A kind of synthetic method of tetrahydroisoindole-1,3-dione derivative
A technology of tetrahydroisoindole and synthesis method is applied in the field of tetrahydroisoindole-1 and achieves the effects of mild reaction conditions, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
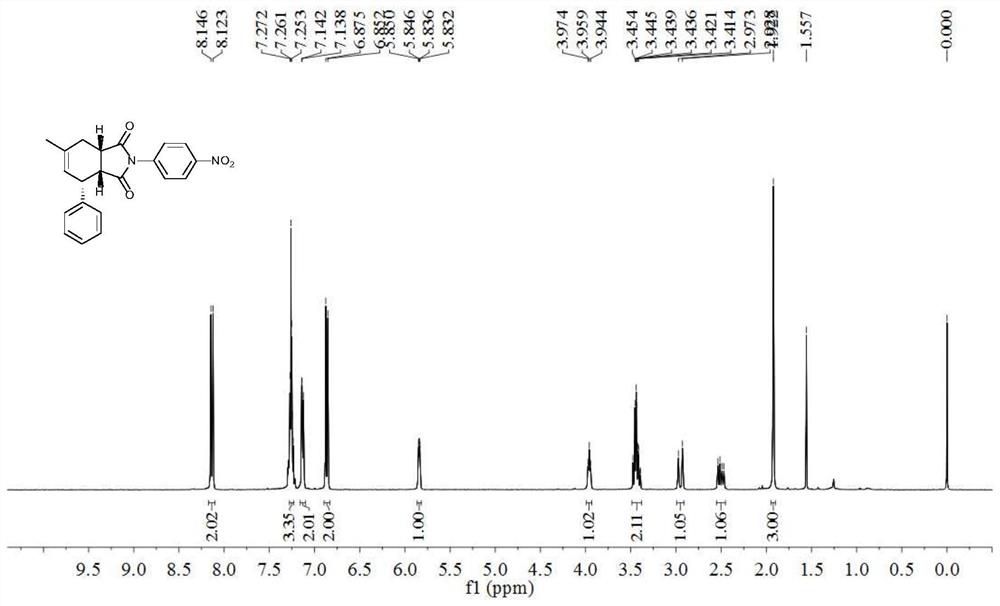
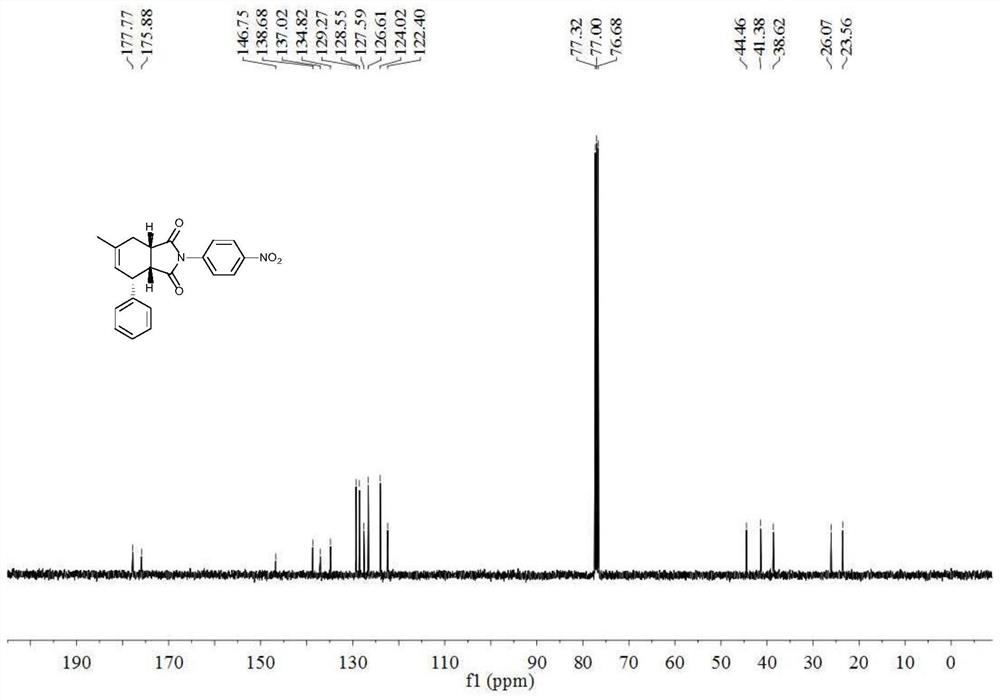
[0024] 6-Methyl-2-(4-nitrophenyl)-4-phenyl-3a,4,7,7a-1H-tetrahydroisoindole-1,3(2H)-dione(±)- Synthesis of ((3aR, 4R, 7aS)-6-methyl-2-(4-nitrophenyl)-4-phenyl-3a, 4, 7, 7a-tetrahydro-1H-isoindole-1, 3(2H)-dione)
[0025] With (3-Methyl-but-2-enyl)-benzene (compound 1a), N-(4-nitrophenyl) maleimide (compound 2) as raw material and in 2,3-dichloro-5 , The reaction under the oxidation of 6-dicyano-p-benzoquinone produces 6-methyl-2-(4-nitrophenyl)-4-phenyl-3a, 4,7,7a-1H-tetrahydroisoindole -1,3(2H)-diketone (compound 3a), its chemical reaction formula is:
[0026]
[0027] The method is specifically:
[0028] Under the protection of inert gas (nitrogen or argon), (3-Methyl-but-2-enyl)-benzene (0.40mmol), 2,3-dichloro-5,6-dicyano-p-benzoquinone (0.3mmol ), N-(4-nitrophenyl)maleimide (0.20mmol) was added to a Schlenk bottle, nitrogen was replaced three times, 2mL of chlorobenzene was added (calcium hydride was distilled to remove water), heated to 110°C for 60h, TLC plate mo...
Embodiment 2
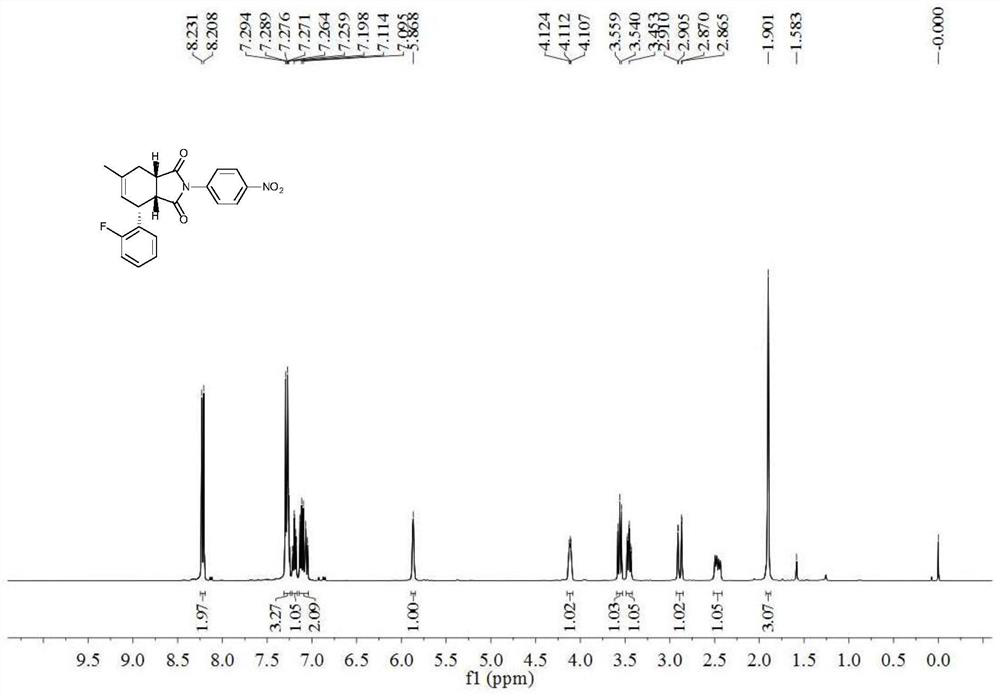
[0034]6-Methyl-2-(4-nitrophenyl)-4-(2-fluorophenyl)-3a,4,7,7a-1H-tetrahydroisoindole-1,3(2H)-di Ketone (±)-((3aR,4R,7aS)-4-(2-fluorophenyl)-6-methyl-2-(4-nitrophenyl)-3a,4,7,7a-tetrahydro-1H-isoindole-1, Synthesis of 3(2H)-dione)
[0035] With 1-Fluoro-2-(3-methyl-but-2-enyl)-benzene (compound 1b), N-(4-nitrophenyl) maleimide (compound 2a) as raw material and in 2, 6-Methyl-2-(4-nitrophenyl)-4-(2-fluorophenyl-3a,4,7 was prepared by oxidation reaction of 3-dichloro-5,6-dicyano-p-benzoquinone , 7a-1H-tetrahydroisoindole-1,3(2H)-dione (compound 3b), its chemical reaction formula is:
[0036]
[0037] The method is specifically:
[0038] Under the protection of inert gas (nitrogen or argon), 1-Fluoro-2-(3-methyl-but-2-enyl)-benzene (0.30mmol), 2,3-dichloro-5,6-dicyano Add p-benzoquinone (0.2mmol), N-(4-nitrophenyl)maleimide (0.20mmol) into a Schlenk bottle, replace nitrogen three times, add 2mL chlorobenzene (calcium hydride distilled to remove water), heat to Reacted at 1...
Embodiment 3
[0044] 6-methyl-2-(4-nitrophenyl)-4-(2-chlorophenyl)-3a,4,7,7a-1H-tetrahydroisoindole-1,3(2H)-di Ketone (±)-((3aR,4R,7aS)-4-(2-chlorophenyl)-6-methyl-2-(4-nitrophenyl)-3a,4,7,7a-tetrahydro-1H-isoindole-1, Synthesis of 3(2H)-dione)
[0045] With 1-Chloro-2-(3-methyl-but-2-enyl)-benzene (compound 1c), N-(4-nitrophenyl) maleimide (compound 2) as raw material and in 2, The reaction under the oxidation of 3-dichloro-5,6-dicyano-p-benzoquinone produced 6-methyl-2-(4-nitrophenyl)-4-(2-chlorophenyl)-3a,4, 7,7a-1H-tetrahydroisoindole-1,3(2H)-dione (compound 3c), its chemical reaction formula is:
[0046]
[0047]
[0048] The method is specifically:
[0049] Under the protection of inert gas (nitrogen or argon), 1-Chloro-2-(3-methyl-but-2-enyl)-benzene (0.50mmol), 2,3-dichloro-5,6-dicyano Add p-benzoquinone (0.4mmol), N-(4-nitrophenyl)maleimide (0.20mmol) into a Schlenk bottle, replace nitrogen three times, add 2mL of chlorobenzene (calcium hydride distilled to remove water),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com