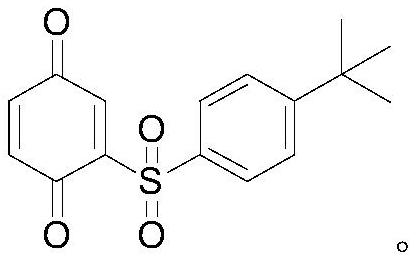
Magnetic silicon-lithium catalyst as well as preparation method and application thereof
A lithium catalyst and catalyst technology, applied in the field of chemical materials, can solve the problems of insufficient green economy, high cost, and large environmental pollution, and achieve the effects of good reaction activity and selectivity, less loss of active components, and high catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 magnetic silicon lithium catalyst
[0042] Weigh 4.0g magnetic Fe 3 o 4 Put into 160mL ethanol-water mixture (ethanol / water=7 / 1), ultrasonically disperse for 30min, add 10mL tetraethyl orthosilicate and 10mL ammonia water to the mixture, stir mechanically for 20h; filter with suction, wash with deionized water 3 times , and then washed the precipitate with absolute ethanol for 3 times, and dried in vacuum at 100°C for 24 hours to obtain Fe 3 o 4 @SiO 2 ; Add 0.8g lithium chloride, 1.5g Fe in the flask 3 o 4 @SiO 2 React with 20mL methanol at 75°C for 12h at reflux. After the reaction, cool to room temperature, remove excess methanol by rotary evaporation, filter with suction, wash the precipitate with absolute ethanol 3 times, and vacuum dry at 90°C for 24h to obtain the silicon lithium catalyst.
[0043] figure 1 The SEM image of the prepared magnetic silicon-lithium catalyst shows that the surface morphology of the silicon-lithi...
Embodiment 2
[0044] The preparation of embodiment 2 magnetic silicon lithium catalysts
[0045] Weigh 4.0g magnetic Fe 3 o 4 Put into 160mL ethanol-water mixture (ethanol / water=7 / 1), ultrasonically disperse for 30min, add 10mL tetraethyl orthosilicate and 10mL ammonia water to the mixture, stir mechanically for 20h; filter with suction, wash with deionized water 3 times , and then washed the precipitate with absolute ethanol for 3 times, and dried in vacuum at 100°C for 24 hours to obtain Fe 3 o 4 @SiO 2 ; Add 0.9g lithium chloride, 1.8g Fe in the flask 3 o 4 @SiO 2 React with 75mL of methanol at 50°C for 72h at reflux. After the reaction, cool to room temperature, remove excess methanol by rotary evaporation, filter with suction, wash the precipitate with absolute ethanol 6 times, and dry in vacuum at 80°C for 72h to obtain the silicon-lithium catalyst. The preparation of embodiment 3 magnetic silicon lithium catalysts
Embodiment 3
[0046] Weigh 4.0g magnetic Fe 3 o 4 Put into 160mL ethanol-water mixture (ethanol / water=7 / 1), ultrasonically disperse for 30min, add 10mL tetraethyl orthosilicate and 10mL ammonia water to the mixture, stir mechanically for 20h; filter with suction, wash with deionized water 3 times , and then washed the precipitate with absolute ethanol for 3 times, and dried in vacuum at 100°C for 24 hours to obtain Fe 3 o 4 @SiO 2 ; Add 1.0g lithium chloride, 2.0g Fe in the flask 3 o 4 @SiO 2 and 55mL methanol, reflux at 110°C for 72h, after the reaction, cool to room temperature, remove excess methanol by rotary evaporation, filter with suction, wash the precipitate with absolute ethanol 3 times, and vacuum dry at 90°C for 24h to obtain the silicon-lithium catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com