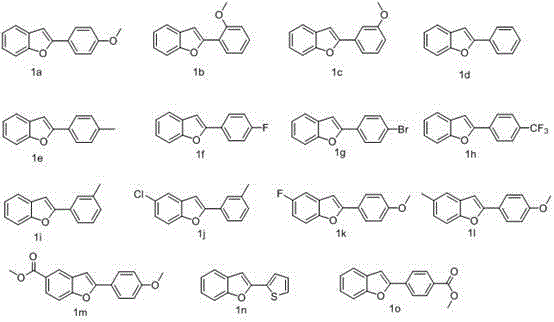
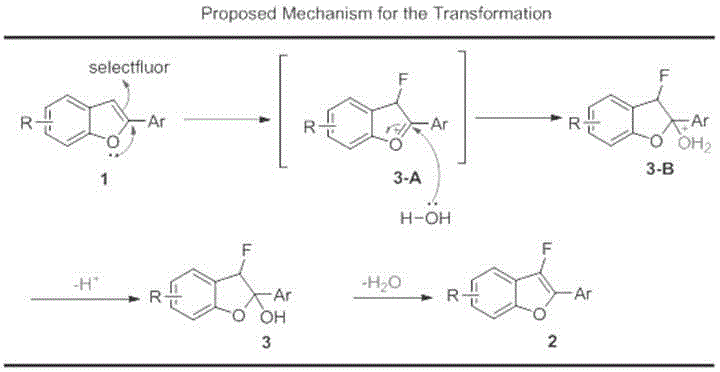
Synthesis method of 2-position aryl substituted benzofuran ring 3-position fluoro
A benzofuran and aryl technology, applied in the field of fluorine organic compounds, can solve the problems of harsh reaction conditions, failure to reflect the application value of organic synthesis, and low yield, and achieve extremely easy operation, good chemical selectivity and functional group Compatibility, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 3-fluoro-2-(4-methoxyphenyl)benzofuran (2a)
[0033] 2-(4-Methoxyphenyl)benzofuran (1a, 112 mg, 0.5 mmol) was dissolved in MeCN (8.0 mL), and SelectFluor (195 mg, 0.55 mmol, 1.1 eq) was added at room temperature (about 25 O c), was added to the solution in water (0.4 ml), then the resulting mixture was stirred at room temperature for 1 h, monitored by TLC (PE:EA = 10:1), once the reaction was complete, the solution in EA (30 ml), and water , washed with brine and dried (Na2SO4), the solvent was removed under reduced pressure, and the residue was used directly in the next step; the residue was dissolved in dry dichloromethane (5 mL), and pyridine (0.41 mL, 5 millimoles, 10 equivalents), and then dropwise added thionyl chloride (0.75 millimoles, 1.5 equivalents, 0.055 ml) solution at this temperature, then the reaction mixture was stirred at room temperature, monitored by TLC overnight, the reaction was complete, and then DCM (10 mL) solution was added, was...
Embodiment 2
[0035] Synthesis of 3-fluoro-2-(2-methoxyphenyl)benzofuran (2b)
[0036] The method is the same as the synthesis of 2a to obtain a colorless oily liquid with a yield of 71%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.70-7.68 (m, 1H), 7.63 (d, J =7.2 Hz, 1H), 7.49 (d, J =8.0 Hz, 1H), 7.44-7.39 (m, 2H), 7.36-7.28 (m, 2H), 7.10 (t, J =7.2 Hz, 1H), 7.03 (d, J =8.0 Hz, 1H), 3.87 (s, 3H) ppm; 13 C NMR (100 MHz, CDCl 3 ) δ 156.75, 151.75 (d, J =8.8 Hz), 144.22 (d, J =255.1 Hz), 136.29 (d, J =24.1 Hz), 130.44, 129.45 (d, J =2.3 Hz), 124.99, 122.88, 120.69 (d, J =20.1 Hz), 120.68, 117.76 (d, J =4.4 Hz), 117.68 (d, J =3.0 Hz), 111.74, 111.57, 55.81 ppm; 19 F NMR (376 MHz, CDCl 3 ) δ -166.06 ppm; IR : (KBr)ν max 2971, 1599, 1495, 1392, 1258, 1026, 744 cm -1; MS (EI) m / z (%): 242 (M+, 100). HRMS calculated value (calcd for) C 15 h 11 FO 2 : 242.0743; Found: 242.0741.
Embodiment 3
[0038] Synthesis of 3-fluoro-2-(3-methoxyphenyl)benzofuran (2c)
[0039] The method is the same as 2a, and a white solid (melting point: 59-61 O C), yield 62%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.61 (d, J =7.2 Hz, 1H), 7.53 (d, J =8.0 Hz, 1H), 7.49-7.45 (m, 2H), 7.40 (t, J =8.0 Hz, 1H), 7.37-7.27 (m, 2H), 6.93-6.90 (m, 1H), 3.90 (s, 3H) ppm; 13 C NMR (100 MHz, CDCl 3 ) δ 159.90, 151.11 (d, J =9.3 Hz), 144.74 (d, J =256.2 Hz), 137.98 (d, J =20.0 Hz), 129.90, 129.84, 125.47, 123.23, 120.63 (d, J =19.3 Hz), 117.66, 117.37 (d, J =6.2 Hz), 114.27, 111.80, 109.79, 55.34 ppm. 19 F NMR (376 MHz, CDCl 3 ) δ -169.48(d, J =1.88 Hz)IR :(KBr)ν max 3066, 1643, 1456, 1345, 1291, 1048, 750cm -1 ; MS (EI) m / z (%): 242 (M+, 100). HRMS calculated value (calcd for) C 15 h 11 FO 2 : 242.0743; Found: 242.0748.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com