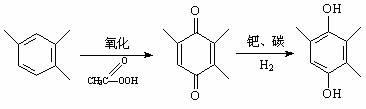
Method for synthesizing 2,3,5-trimethylbenzoquinone and 2,3,5-trimethylhydroquinone
A technology of trimethylhydroquinone and trimethylbenzoquinone, which is applied in chemical instruments and methods, preparation of quinone oxides, preparation of organic compounds, etc., can solve the problems of high technical requirements and short process, and achieve simple steps, The effect of low equipment requirements and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 2g of 2,3,6-trimethylphenol in 20ml of acetonitrile, add it into a three-necked flask, weigh 0.1g of Cu-SBA-15 mesoporous molecular sieve into the flask, heat it in a water bath to 50°C, add 3ml of 30% Hydrogen peroxide, start timing, react for 3h. The final conversion rate was 6.6%, the selectivity to TMBQ was 62.0%, and the selectivity to TMHQ was 18.9%.
Embodiment 2
[0028] Dissolve 2g of 2,3,6-trimethylphenol in 20ml of acetonitrile, add it into a three-necked flask, weigh 0.1g of Cu-SBA-15 mesoporous molecular sieve into the flask, heat it in a water bath to 60°C, add 3ml of 30% Hydrogen peroxide, start timing, react for 3h. The final conversion rate was 12.0%, the selectivity to TMBQ was 59.4%, and the selectivity to TMHQ was 18.9%.
Embodiment 3
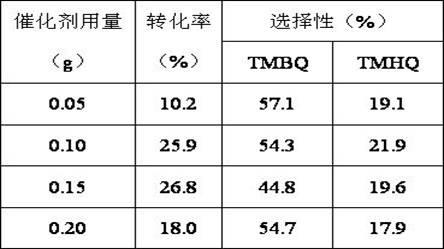
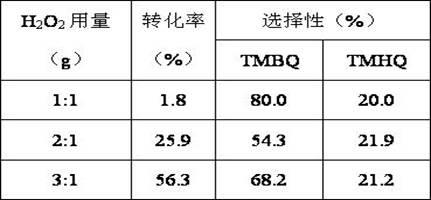
[0030] Dissolve 2g of 2,3,6-trimethylphenol in 20ml of acetonitrile, add it into a three-necked flask, weigh 0.1g of Cu-SBA-15 mesoporous molecular sieve into the flask, heat it in a water bath to 70°C, add 3ml of 30% Hydrogen peroxide, start timing, react for 3h. The final conversion rate was 25.9%, the selectivity to TMBQ was 54.3%, and the selectivity to TMHQ was 21.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com