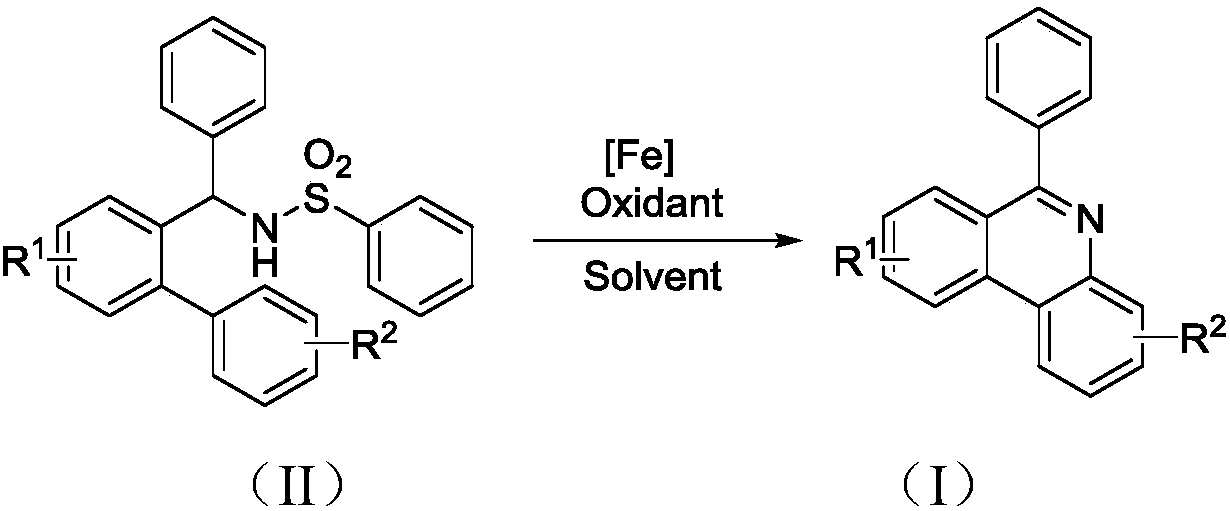
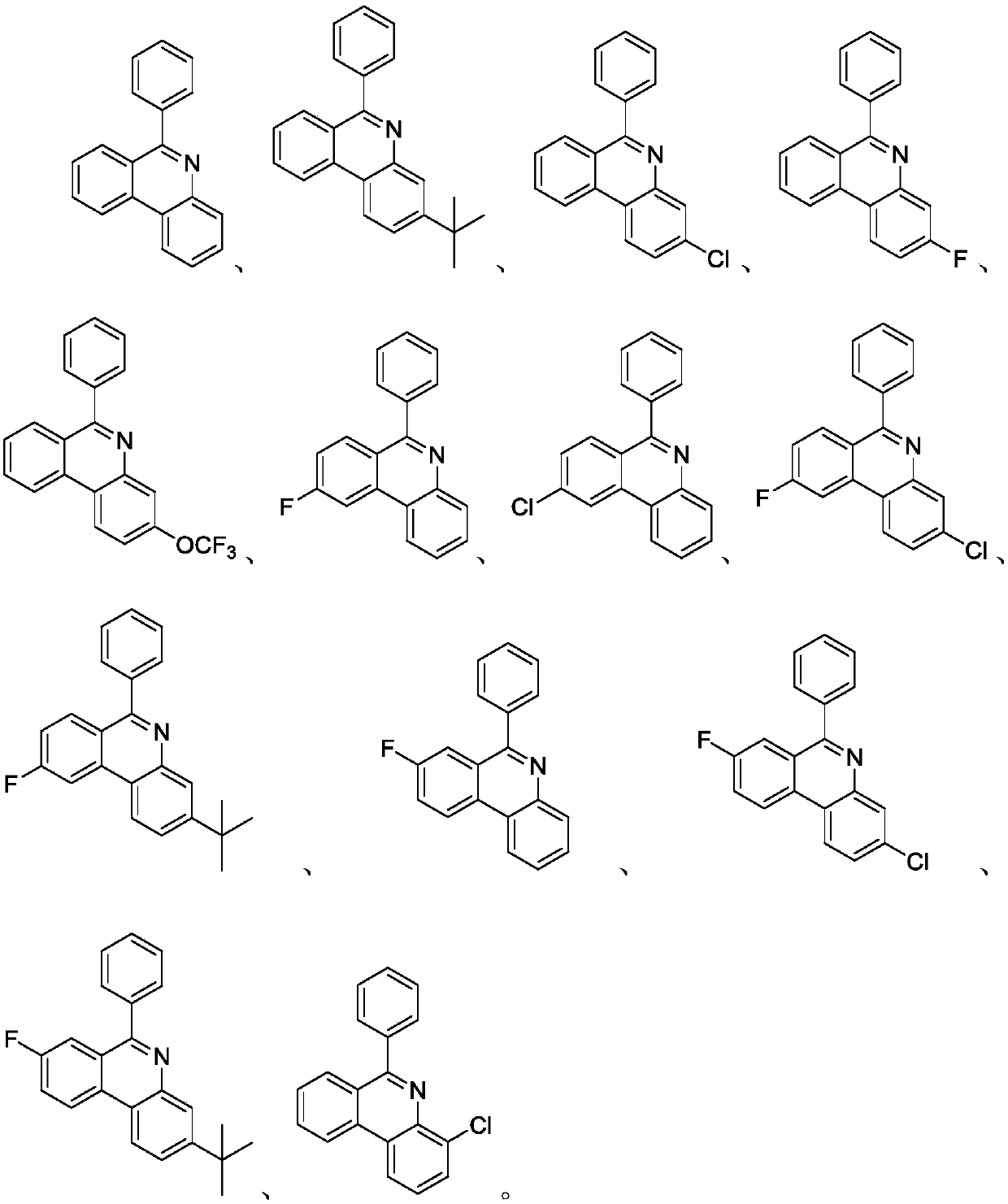
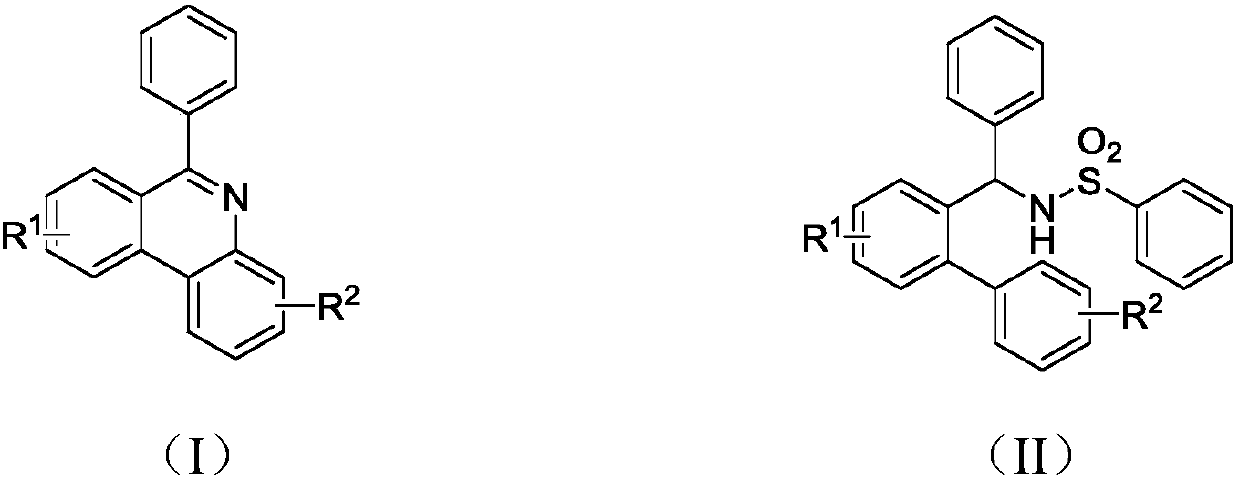Synthesis method of 6-substituted phenanthridine compound
A synthesis method and compound technology, applied in the field of organic compound synthesis, can solve the problems of high reaction temperature control requirements, limited compound synthesis, and high reaction temperature requirements, and achieve the effects of simple raw materials, simple operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Add 119.7mg (0.3mmol) of N-[1-phenyl-1'-biphenyl]benzenesulfonamide, 212.6mg (0.6mmol) of Selectfluor and 1.7mg (0.03mmol) of Fe powder into a 25mL thick-walled pressure-resistant tube , acetonitrile as solvent, the dosage is 3mL. Next, it was magnetically stirred at 80° C. for 12 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 8mL of dichloromethane and 8mL of water to extract simultaneously, extract 3 times, get the organic layer containing dichloromethane and target product each time, i.e. the extract, and in the last Add 239.4 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether and ethyl acetate is 10 : 1 mixed solution was eluted as eluent, the eluate containing target product was collected, and the solvent was evaporated to obtain a...
Embodiment 2
[0027]
[0028] 136.5mg (0.3mmol) N-[1-phenyl-1'-biphenyl (4'-tert-butyl)] benzenesulfonamide, 204.3mg (0.9mmol) DDQ and 5.7mg (0.045mmol) FeCl 2Add it to a 25mL thick-walled pressure-resistant tube, using 1,2-dichloroethane as a solvent, and the dosage is 1.5mL. Next, it was magnetically stirred at 90° C. for 24 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 3mL ethyl acetate and 8mL water to extract simultaneously, extract 3 times, get the organic layer containing ethyl acetate and the target product each time, i.e. the extract, and in the last Add 273 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether and ethyl acetate is 10: The mixed solution of 1 was eluted as an eluent, and the eluate containing the target product was collected, and the solvent ...
Embodiment 3
[0031]
[0032] 129.9mg (0.3mmol) of N-[1-phenyl-1'-biphenyl(4'-chloro)]benzenesulfonamide, 645.5mg (1.05mmol) of Oxone and 26.6mg (0.09mmol) of FeBr 3 Add it to a 25mL thick-walled pressure-resistant tube, using 1,4-dioxane as a solvent, and the dosage is 3mL. Next, magnetic stirring was performed at 130° C. for 36 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 15mL of dichloromethane and 8mL of water to extract at the same time, extract 3 times, each time get the organic layer containing dichloromethane and target product, i.e. the extract, and in the last Add 259.8 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether to ethyl acetate is 10 : The mixed solution of 1 was eluted as the eluent, and the eluate containing the target product was collected, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com