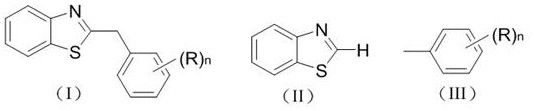
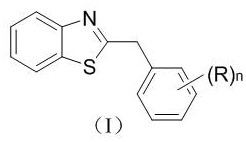
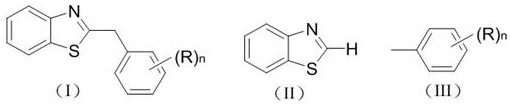
Photocatalytic synthesis method of C2 substituted 2H-benzothiazole benzylated derivative
A technology of benzothiazole benzyl and synthesis method is applied in the field of photocatalytic synthesis of C2-substituted 2H-benzothiazole benzyl derivatives, and can solve the problems of oxidant organic peroxides, such as inflammability and explosion, high reaction temperature and the like , to achieve the effect of low price, good reaction repeatability and simple catalytic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Synthesis of Derivative Ia (n=0)
[0022] Weigh 2 H -Benzothiazole (0.3 mmol, 40.6 mg), toluene 6.0 mmol, 552.8 mg), Selectfluor (0.9 mmol, 318.9 mg) and trifluoroacetic acid (0.30 mmol, 34.2 mg) in a 25 mL reaction tube, then added acetonitrile ( 58 mmol, 2.4 g), placed under the irradiation of 30 W LED blue light to react, stirred at room temperature, monitored by TLC, after about 16 h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (washing The solvent removal is petroleum ether-ethyl acetate mixed solvent with a volume ratio of 1:10) to obtain a white solid, i.e. derivative Ia. Yield 78%.
[0023] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0024] 1 H NMR (500 MHz, CDCl 3 ) δ 7.95 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.0 Hz,1H), 7.46 (t, J = 7.5Hz, 1H), 7.29 – 7.21 (m, 6H), 4.39 (s, 2H). 13 C NMR (125MHz...
Embodiment 2
[0026] Example 2 Synthesis of derivative Ib (n=1, (R)n=p-methyl)
[0027] Weigh 2 H- Benzothiazole (0.3 mmol, 40.6 mg), p-xylene (6.0 mmol, 636.3 mg), Selectfluor (0.9 mmol, 318.9 mg) and trifluoroacetic acid (0.30 mmol, 34.2 mg) in a 25 mL reaction tube, and then Add acetonitrile (65 mmol, 2.7 g), react under the irradiation of 20 W LED blue light, stir the reaction at room temperature, monitor by TLC, the reaction ends after about 16 h, the reaction solution is concentrated to remove the solvent, and the concentrated solution is subjected to column chromatography Separation (eluent is a mixed solvent of petroleum ether-ethyl acetate with a volume ratio of 1:10) to obtain a white solid, namely derivative Ib. Yield 86%.
[0028] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0029] 1 H NMR (500 MHz, CDCl 3 ) δ 7.98 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 8.0 Hz,1H), 7.42 (t, J = 7.5 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 7.5 Hz,2H...
Embodiment 3
[0031] Example 3 Synthesis of derivative Ic (n=2, (R)n=3, 5-dimethyl)
[0032] Weigh 2 H - Benzothiazole (0.3 mmol, 40.6 mg), mesitylene (4.5 mmol, 540.4 mg), Selectfluor (0.6 mmol, 212.6 mg) and trifluoroacetic acid (0.24 mmol, 27.4 mg) in a 25 mL reaction tube, and then Add acetonitrile (45 mmol, 1.9 g), place it under a 20 W LED blue light to react, stir the reaction at room temperature, monitor with TLC, after about 16 h, the reaction ends, the reaction solution is concentrated to remove the solvent, and the concentrated solution is separated by column chromatography (washing The solvent removal is petroleum ether-ethyl acetate mixed solvent with a volume ratio of 1:10) to obtain a white solid, namely the derivative Ic. The yield is 83%.
[0033] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0034] 1 H NMR (500 MHz, CDCl 3 ) δ 8.05 (d, J = 8.2 Hz, 1H), 7.83 (d, J = 8.0 Hz,1H), 7.39 (t, J = 8.0 Hz, 1H), 7.25 (t, J = 7.5 Hz, 1H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com