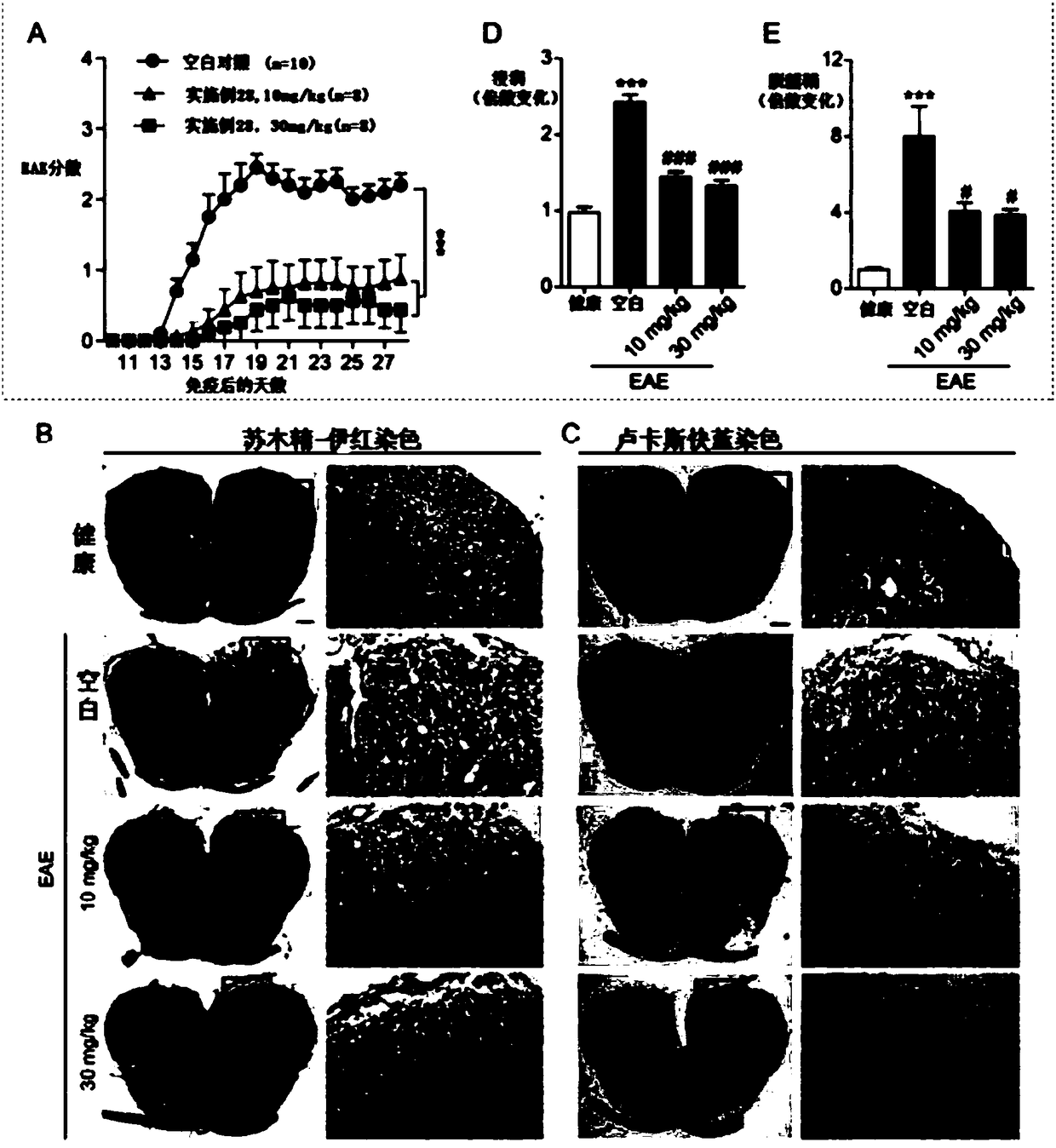
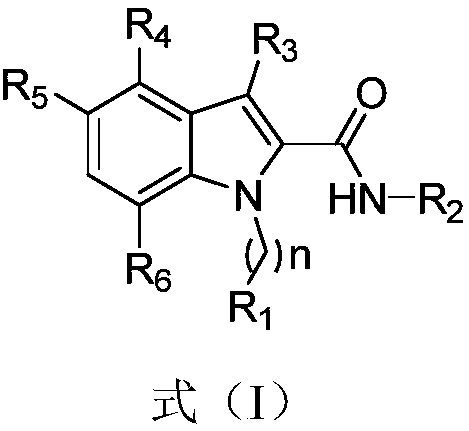
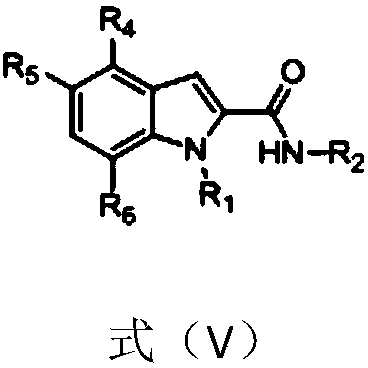
1H-indole-2-carboxamide derivative and preparation method and applications thereof
A technology of formamide and derivatives, applied in the field of medicine, can solve problems such as no marketed drugs yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: N-(adamantan-1-yl)-1H-indole-2-carboxamide (1)
[0126] (a) Preparation of 1H-indole-2-carboxylic acid 1a Ethyl-1H-indole-2-carboxylate (1.0g, 5.3mmol) was added to 10ml of water, and then potassium hydroxide (2.0g , 31.8mmol), refluxed at 120°C for 0.5 hour, cooled to room temperature, adjusted pH to 3 to 4 with 10M hydrochloric acid, a large amount of white solid precipitated, filtered, and the filter cake was dried to obtain 853mg (99.9%) of white solid.
[0127] (b) Preparation of N-(adamantan-1-yl)-1H-indole-2-carboxamide (1) 1a (500mg, 3.1mmol) was added to 5ml of dichloromethane, and a catalytic amount of DMF was added , then add oxalyl chloride (787mg, 6.2mmol), stir at room temperature for 1 hour, spin dry the system, add amantadine (562mg, 3.72mmol), triethylamine (1.25g, 12.4mmol) into 5ml of dichloromethane, Stir at room temperature for 8 hours, spin the system to dryness, and purify by silica gel column chromatography (dichloromethane) to obtain...
Embodiment 2
[0128] Example 2: Preparation of N-(adamantan-1-yl)-1-pentyl-1H-indole-2-carboxamide (2) (1) (150 mg, 0.51 mmol) was added to 4 ml of anhydrous DMF In, add NaH (40mg, 0.77mmol), stir at room temperature for 15 minutes, then add 1-bromopentane, stir at room temperature for 3 hours, add 15ml of water to quench the reaction, then extract with ethyl acetate (20ml×3), combine The organic layer was washed with water (30ml×3), and then washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, the organic layer was spin-dried, and purified by silica gel column chromatography (dichloromethane) to obtain (2) 135 mg of a white solid ( 73%) 1 H NMR (400MHz, CDCl 3 )δ7.59(d, J=7.9Hz, 1H), 7.36(d, J=8.4Hz, 1H), 7.30–7.23(m, 1H), 7.11(t, J=7.4Hz, 1H), 6.73( s,1H),5.90(s,1H),4.51(t,J=7.3Hz,2H),2.14(d,J=8.9Hz,9H),1.81–1.67(m,8H),1.34–1.23(m ,4H),0.87(t,J=6.8Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ162.11, 138.13, 133.40, 126.20, 123.58, 121.70, 120.28, 110.43, 103....
Embodiment 3
[0129] Example 3: N-(Adamantan-1-yl)-5-methoxy-1H-indole-2-carboxamide (3)
[0130] (a) Preparation of 5-methoxy-1H-indole-2-carboxylic acid 3a The preparation method of 3a is the same as that of 1a, except that ethyl-5-methoxy-1H-indole is used Ethyl-1H-indole-2-carboxylate (500 mg, 2.3 mmol) was substituted for ethyl-1H-indole-2-carboxylate to afford 340 mg (77%) of 3a as a white solid.
[0131] (b) the preparation of N-(adamantan-1-yl)-5-methoxy-1H-indole-2-carboxamide (3) The preparation method of (3) is the same as the preparation method of (1), different The difference is that 3a (340mg, 1.78mmol) was used instead of 1a to give (3) 100mg (17.4%) pale yellow solid 1 H NMR (400MHz, CDCl 3)δ10.23(s,1H),7.43(d,J=8.9Hz,1H),7.03(d,J=1.8Hz,1H),6.93(dd,J=8.9,2.3Hz,1H),6.69( d,J=1.1Hz,1H),5.92(s,1H),3.84(s,3H),2.17(d,J=10.2Hz,9H),1.83–1.68(m,6H). 13 C NMR (101MHz, CDCl 3 )δ161.13, 154.57, 132.45, 132.02, 128.05, 115.28, 113.22, 102.24, 100.96, 55.82, 52.58, 41.93, 36.46, 29....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com