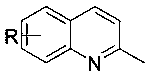
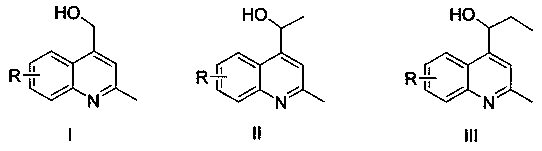
Preparation method of 2-methyl-4-hydroxymethyl quinoline and derivatives thereof
A technology of hydroxymethylquinoline and methylquinoline, which is applied in the field of preparation of 2-methyl-4-hydroxymethylquinoline derivatives, can solve the problems of low universality and poor functional group tolerance, etc. Achieve the effects of strong controllability, less side reaction products and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
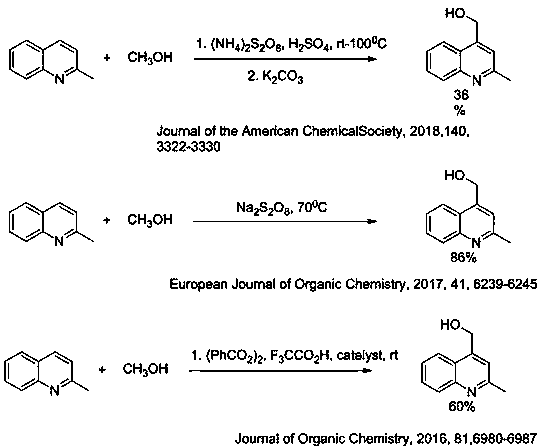
[0030] 2-Methylquinoline (1 mmol, 143 mg), Selectfluor (2 mmol, 0.7 g) and AgNO 3 (1mmol, 167mg) was added to an aqueous solution of methanol (10ml, MeOH:H 2 O=4:1) and reacted at 80°C for 3h, neutralized with saturated sodium bicarbonate solution, extracted with ethyl acetate (3*20 mL), combined organic layers, washed with saturated brine (20 mL), dried over anhydrous sodium sulfate , filtered and concentrated. Column chromatography (eluent: ethyl acetate / n-hexane = 3:2) yielded 140 mg of the product 2-methyl-4-hydroxymethylquinoline with a yield of 81%.
[0031] 1 H NMR (600MHz, CDCl 3 ) δ ppm 8.02 (d, J = 8.4 Hz, 1H), 7.86 (d, J =8.2 Hz, 1H), 7.68 – 7.62 (m, 1H), 7.47 (t, J = 7.5 Hz, 1H), 7.41 ( s, 1H), 5.18 (s, 2H), 2.64 (s, 3H).; 13 C NMR (151 MHz, CDCl 3 ) δ ppm 158.95, 147.12, 146.77, 129.38, 128.68, 125.85, 124.06, 122.66, 119.07, 61.17, 25.10.; HRMS(ESI) Calcd. for C 11 h 12 NO [(M+H) + ] 174.0913, found 174.0912.
Embodiment 2
[0033] 2-Methyl-6-fluoroquinoline (1 mmol, 161 mg), Selectfluor (2 mmol, 0.7 g) and AgNO 3 (1mmol, 167mg) was added to an aqueous solution of methanol (10ml, MeOH:H 2 O=4:1) and reacted at 80°C for 3h, neutralized with saturated sodium bicarbonate solution, extracted with ethyl acetate (3*20 mL), combined organic layers, washed with saturated brine (20 mL), dried over anhydrous sodium sulfate , filtered and concentrated. Column chromatography (eluent: ethyl acetate / n-hexane = 3:2) yielded 172 mg of the product 2-methyl-4-hydroxymethyl-6-fluoroquinoline with a yield of 90%.
[0034] 1 H NMR (600MHz, CDCl 3 ) δ ppm 8.03 (dd, J = 9.2, 5.5 Hz, 1H), 7.51 (dd, J = 9.8, 2.8 Hz, 1H), 7.46 – 7.41 (m, 2H), 5.12 (s, 2H), 2.71 (s , 3H).; 13 CNMR (151 MHz, CDCl 3 ) Δ PPM 160.81, 159.18, 158.32, 158.30, 145.52, 144.63,131.44, 131.38, 124.80, 124.73, 119.86, 119.18, 106.54,61.57, 25.19.; 11 h 11 FNO [(M+H) + ] 192.0819, found 192.0819.
Embodiment 3
[0036] 2-Methyl-6-chloroquinoline (1 mmol, 177 mg), Selectfluor (2 mmol, 0.7 g) and AgNO 3 (1mmol, 167mg) was added to an aqueous solution of ethanol (10ml, EtOH:H 2 O=4:1) and reacted at 80°C for 3h, neutralized with saturated sodium bicarbonate solution, extracted with ethyl acetate (3*20 mL), combined organic layers, washed with saturated brine (20 mL), dried over anhydrous sodium sulfate , filtered and concentrated. Column chromatography (eluent: ethyl acetate / n-hexane = 1:1) yielded 108 mg of the product 2-methyl-4-(1-ethanol-yl)-6-chloroquinoline with a yield of 49%.
[0037] 1 H NMR (600MHz, CDCl 3 ) δ ppm 7.86 (dd, J = 5.6, 3.1 Hz, 2H), 7.53 (dd, J = 9.0, 2.2 Hz, 1H), 7.41 (s, 1H), 5.45 (q, J = 6.5 Hz, 1H), 4.23 – 4.17 (m,1H), 2.60 (s, 3H), 1.59 (d, J = 6.6 Hz, 3H).; 13 C NMR (151 MHz, CDCl 3 for C 12 h 13 ClNO [(M+H) + ] 222.0680, found 222.0682.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com