Synthetic method of 4-fluoroisoquinoline-5-amine
A technique for the synthesis of fluoroisoquinoline, which is applied in organic chemistry and other fields, can solve the problems of lack of suppliers and high price of 4-fluoroisoquinoline, and achieve simple and easy-to-obtain raw materials, convenient operation and post-treatment, and process selection reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
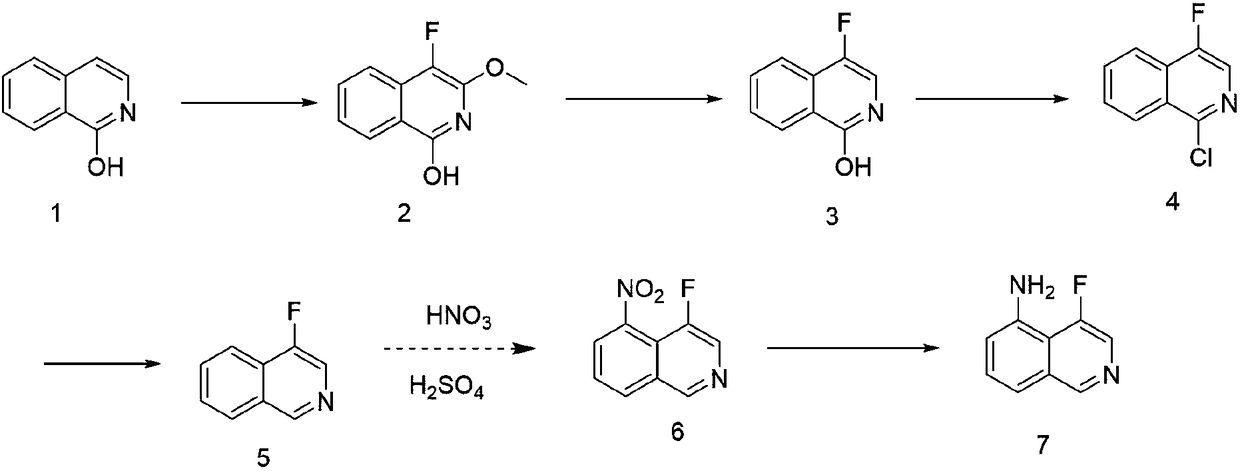
[0049] The first step: the synthesis of 4-fluoro-3-methoxyisoquinolin-1-ol
[0050] Add isoquinolin-1-ol (14.5g, 0.1mol) to MeCN (100ml), MeOH (100ml), Selectfluor (1.3eq), and then heat up and reflux for 3h. Vote for the next step.
[0051] The second step: the synthesis of 4-fluoroisoquinolin-1-ol
[0052] 4-fluoro-3-methoxyisoquinolin-1-ol (0.1mol) was dissolved in 0.5NHCl.aq (100ml), EA (100ml), stirred overnight at 40°C, extracted with EA, and the product was obtained by column chromatography ( 13 g, 80%).
[0053] The third step: the synthesis of 1-chloro-4-fluoroisoquinoline
[0054] 4-Fluoroisoquinolin-1-ol (33g, 0.2mol) was added to POCl3 (150ml) in batches, and the temperature was raised to reflux for 3h. After the reaction was completed, the excess POCl 3 , Na 2 CO 3 aq. Adjust the pH, extract with EA and spin dry column chromatography to obtain the product (25 g, 70%).
[0055] The fourth step: the synthesis of 4-fluoroisoquinoline
[0056] 1-Chloro-4-fluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com