Preparation method of fluoride chalcone
A technology of fluorinated chalcones and fluorinated chalcones, which is applied in the field of preparation of fluorinated chalcones, can solve the problems of high toxicity of fluorinated reagents, difficulty in controlling the degree of reaction, complex synthesis process, etc., and achieves low price and saving The effect of economic cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] A kind of preparation method of fluorinated chalcone of the present invention, its reaction formula is as follows:
[0017]
[0018] First, mix acetonitrile and methanol at a volume ratio of 8:1-10:1 to make a reaction solvent, then add chalcone and electrophilic fluorinating reagent Selectfluor, stir and heat up to 65-75°C for reaction, after the reaction Cool to room temperature, evaporate to dryness to remove the reaction solvent, and then separate the product by column chromatography (V (ethyl acetate): V (petroleum ether) is 20:1), and dry to obtain fluorinated chalcone.
Embodiment 1
[0020] Step 1: Mix 450mL of acetonitrile and 50mL of methanol to make a reaction solvent.
[0021] Step 2: Add 20.8g chalcone and 70g Selectfluor into the reactor, raise the temperature to 65°C, heat and stir for 8h.
[0022] Step 3: Cool the reaction mixture to room temperature, spin dry, separate by column chromatography and evaporate to dryness to obtain 13.6 g of fluorinated chalcone.
[0023] The molecular formula of the resulting product fluorinated chalcones is:
[0024]
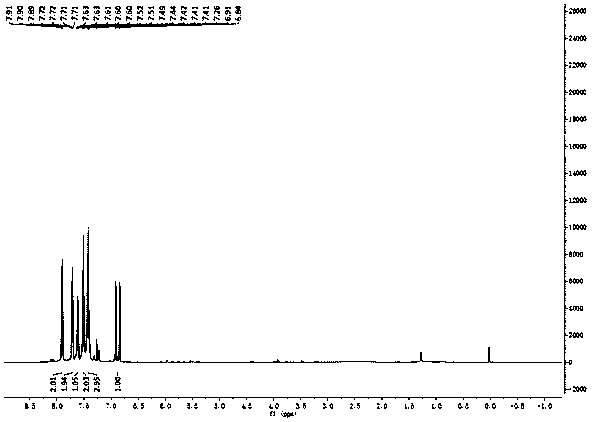
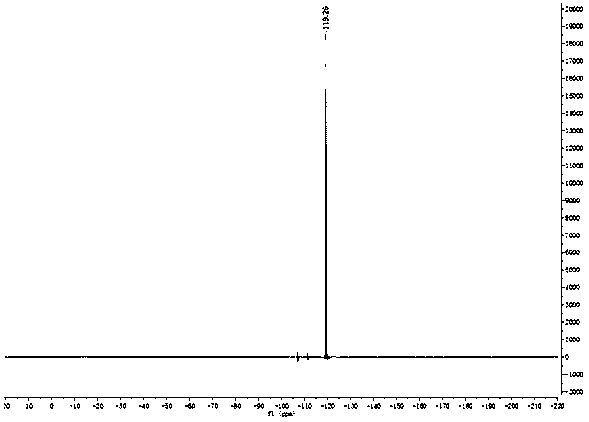
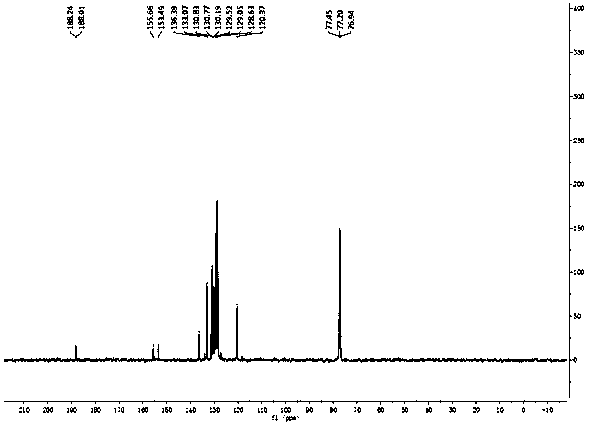
[0025] figure 1 , 2 and 3 are the NMR spectra of the prepared fluorinated chalcones, wherein, figure 1 for 1 H Spectrum, figure 2 for 19 F spectrum, Figure 3 is 13 C spectrum. figure 1 Through the integration and displacement position, it can be seen that the product has 10 hydrogen atoms; figure 2 It can be seen that the product contains a fluorine atom, and by observing the displacement, it can be seen that the fluorine atom is located at the α position; Figure 3 shows that the number o...
Embodiment 2
[0027] Step 1: Mix 400mL acetonitrile and 50mL methanol to make a reaction solvent.
[0028] Step 2: Add 24.2g of 4'-chlorochalcone and 63.7g of Selectfluor into the reactor, raise the temperature to 65°C, and heat and stir for 12 hours.
[0029] Step 3: Cool the reaction mixture to room temperature, spin dry, separate by column chromatography and evaporate to dryness to obtain 15.6 g of fluorinated chalcone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com