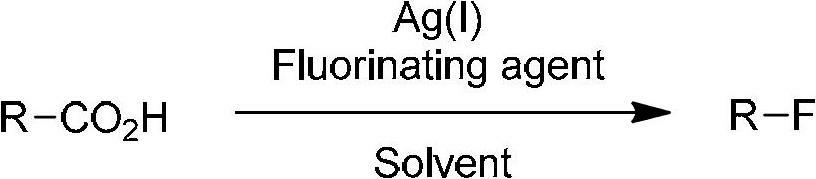
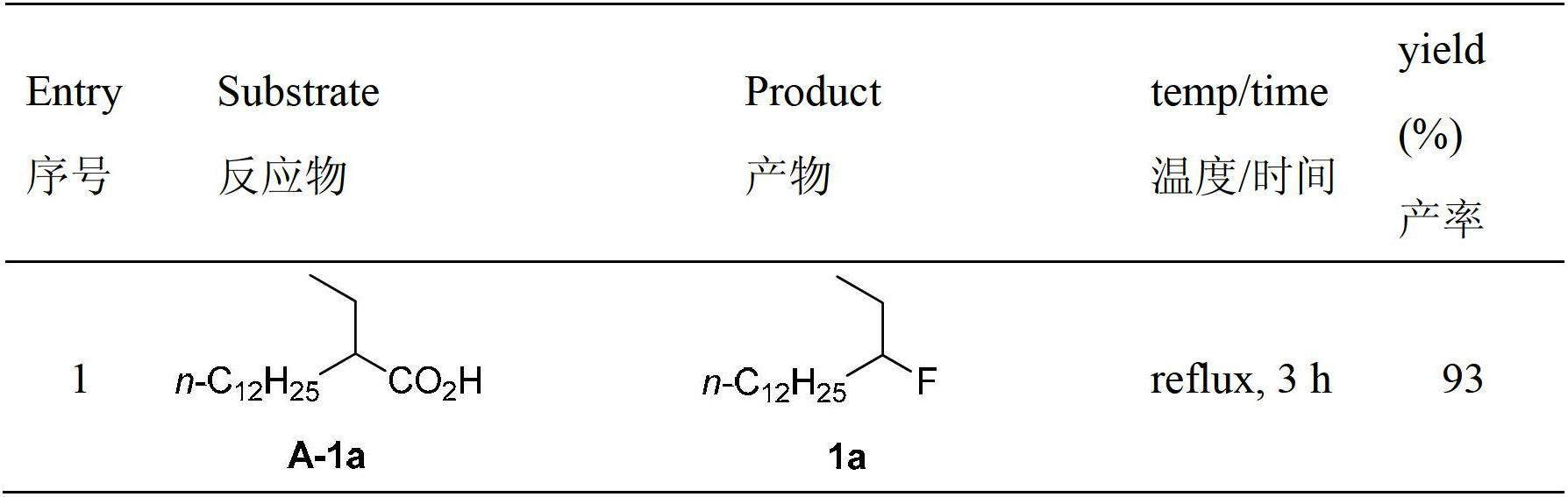
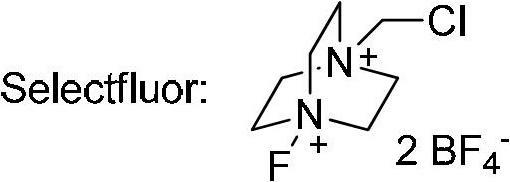Decarboxylation and fluorination method for carboxylic acid
A technology for decarboxylation of fluorinated and carboxylic acids, which is applied to the preparation of carboxylic acid esters, organic chemical methods, chemical instruments and methods, etc., and can solve problems such as low reaction efficiency, large limitations, and failure to reflect the application value of organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 3-fluoropentadecane (1a)
[0040] 2-Ethyltetradecanoic acid (A-1a, 51.2mg, 0.20mmol), AgNO 3 (6.8mg, 0.04mmol) and Selectfluor (141.6mg, 0.4mmol) were sequentially added to the reaction tube. Then 2 ml each of acetone and water were added. The reaction solution was refluxed for 10 hours with stirring. The system was cooled to room temperature, and then extracted with dichloromethane (15 mL×3). The organic phases were combined and dried over anhydrous sodium sulfate. Filtrate, concentrate the filtrate, and purify the crude product by silica gel column chromatography with hexane as the eluent to obtain the product 3-fluoropentadecane as a colorless liquid. Yield: 42.7 mg (93%). IR (neat): ν(cm -1 )2926,2855,1465; 1 H NMR (400MHz, CDCl 3 )δ0.88(3H,t,J=7.2Hz),0.96(3H,t,J=7.6Hz),1.26-1.69(24H,m),4.29-4.49(1H,m); 13 C NMR (100MHz, CDCl 3 )δ9.4(d, J=5.9Hz), 14.1, 22.7, 25.2(d, J=4.4Hz), 28.1(d, J=21.2Hz), 29.37, 29.43, 29.55, 29.56, 29.6, 29.7, 29.8 ,31....
Embodiment 2
[0042] Synthesis of 1,3-dicyclohexyl-2-fluoropropane (1b)
[0043] The method is the same as that of 1a, and the reaction time is 10 hours. It is yellow oily liquid. Yellow oil.IR(neat):ν(cm -1 )2923,1558,1275,1261,764,750; 1 H NMR (400MHz, CDCl 3 )δ0.74-1.76(26H,m),4.52-4.75(1H,m); 13 C NMR (100MHz, CDCl 3 )δ25.2,25.3,25.5,31.9,33.0,33.1(d,J=3.7Hz),42.5(d,J=20.5Hz),89.4(d,J=164.8Hz); 19 F NMR (282MHz, CDCl 3 )δ-177.2(1F,m);EIMS:m / z(rel intensity)206(M-HF,10),178(2),135(8),110(22),96(45),82( 100), 67(33), 55(62), 41(24); HRMS calculated value (calcd for) C 15 h 26 (M-HF): 206.2035, measured value (found) 206.2032.
Embodiment 3
[0045] Synthesis of 1,3-bis(4-chlorophenyl)-2-fluoropropane (1c)
[0046] The method is the same as that of 1a, and the reaction time is 10 hours. It is yellow oily liquid. IR(neat):ν(cm -1 )2925,1492,1275,1261,1091,1016,805,764,750; 1 H NMR (400MHz, CDCl 3 )δ2.73-2.89(4H,m),4.66-4.84(1H,m)7.06(4H,d,J=8.4Hz),7.20(4H,d,J=8.4Hz); 13 C NMR (100MHz, CDCl 3 )δ40.3(d,J=21.2Hz),94.0(d,J=174.3Hz),128.7,130.8,132.7,135.3(d,J=3.6Hz); 19 F NMR (282MHz, CDCl 3 )δ-178.6(1F,m); EIMS:m / z(rel intensity)282(M + ,27),254(4),227(3),192(2),157(17),125(100),101(11),91(14),75(7),51(3);HRMS Calculated value (calcd for) C 15 h 13 Cl 2 F: 282.0378, measured value (found) 282.0380.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com