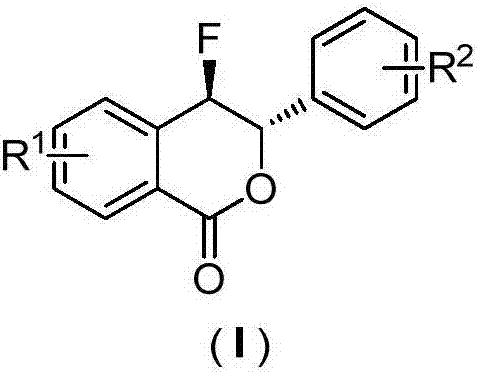
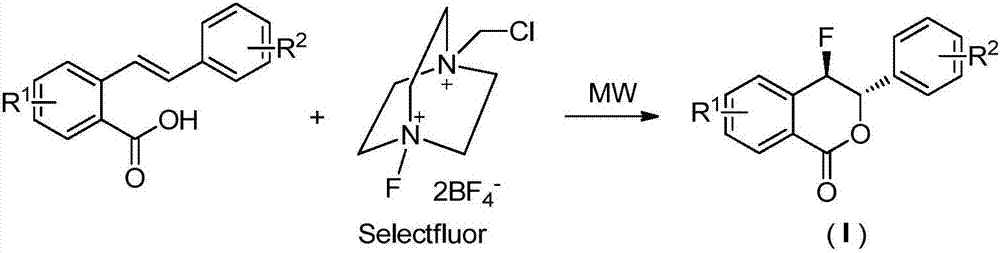
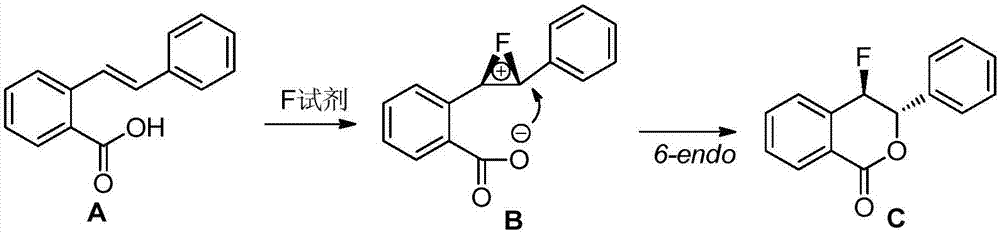
Preparation method of fluoro-3,4-dihydroisocoumarin derivative
A derivative, isocoumarin technology, applied in the direction of organic chemistry, can solve the problems of expensive substrates or catalysts, limited scope of application of substrates, harsh reaction conditions, etc., to achieve green industrial production, easy operation and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1.R 1 =-H, R 2 =-m-CH 3 , the preparation of 4‐fluoro‐3‐(3‐tolyl)‐3,4‐dihydroisocoumarin derivatives
[0026] In a 25mL microwave reaction tube, add 2‐(3‐tolylvinyl)benzoic acid (0.2mmol, 47.6mg), Selectfluor fluorine reagent (0.4mmol, 141.6mg), and 2mL acetonitrile and 2mL water as a mixed solvent, at 100 ℃ for 0.5 h; after the reaction, remove the solvent under reduced pressure, add 10 mL of water to the residue, extract twice with 20 mL of ethyl acetate, wash the extract once with saturated brine; wash the extract with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 3) to obtain 0.047 g of a colorless solid with a yield of 92.0%.
[0027]
[0028] 1 H NMR (400MHz, CDCl 3 )δ:8.16(d,J H‐H =7.6Hz,1H),7.67(t,J H‐H =7.4Hz,1H),7.57‐7.51(m,2H),7.23(t,J H‐H =7.6Hz,1H),7.18-7.12(m,3H),5.80-5.66(m,2H),2.32(s,3H). 13 C NMR (100MHz, CDCl...
Embodiment 2
[0029] Example 2.R 1 =-H, R 2 =-p-NO 2 , the preparation of 4‐fluoro‐3‐(4‐nitrophenyl)‐3,4‐dihydroisocoumarin derivatives
[0030] Add 2‐(4‐nitrostyryl)benzoic acid (0.2mmol, 53.8mg), Selectfluor fluorine reagent (0.3mmol, 106.2mg), and 4mL acetonitrile into a 25mL microwave reaction tube, and react at 80°C 1.0h; After the reaction, remove the solvent under reduced pressure, add 10mL water to the residue, extract twice with 20mL ethyl acetate, wash the extract once with saturated brine; wash the extract with anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 2) to obtain 0.051 g of a colorless solid with a yield of 90.0%.
[0031]
[0032] 1 H NMR (400MHz, CDCl 3 )δ:8.28(d,JH‐H =8.7Hz,2H),8.19(d,J H‐H =7.8Hz,1H),7.75(t,J H‐H =7.5Hz,1H),7.68‐7.58(m,4H),5.81‐5.66(m,2H). 13 C NMR (100MHz, CDCl 3 )δ: 162.5, 148.3, 141.7, 136.1 (d, J F‐C =19.4Hz), 13...
Embodiment 3
[0033] Example 3.R 1 =-Br, R 2 =-H, Preparation of 7-Bromo-4-Fluoro-3-Phenyl-3,4-Dihydroisocoumarin Derivatives
[0034] Add 5‐bromo‐2‐(styryl)benzoic acid (0.2mmol, 60.4mg), Selectfluor fluorine reagent (0.6mmol, 212.4mg), and 4mL DMSO into a 25mL microwave reaction tube, and react at 90°C 1.0h; After the reaction, the solvent was removed under reduced pressure, 10mL of water was added to the residue, extracted twice with 20mL of ethyl acetate, the extract was washed once with saturated brine; the extract was washed with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 3) to obtain 0.058 g of a colorless solid with a yield of 91.0%.
[0035]
[0036] 1 H NMR (400MHz, DMSO) δ: 8.16(s, 1H), 8.00(d, J H‐H =8.1Hz,1H),7.63(d,J H‐H =8.0Hz,1H),7.40‐7.34(m,5H),6.26(dd,J F‐H =47.6Hz,J H‐H =5.6Hz,1H),6.14‐6.10(m,1H). 13 C NMR (100MHz, DMSO) δ: 162.0, 138...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com