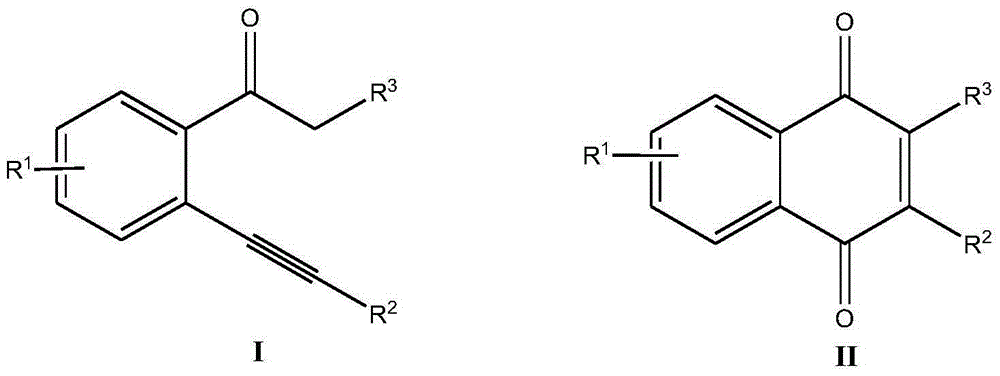
Method for synthesizing 2-substituted-1,4-naphthoquinone compound
A compound, naphthoquinone technology, applied in the field of organic compound synthesis, can solve the problems of polluted transition metal catalyst, unrecyclable, single source of raw materials, etc., and achieves low cost and low toxicity of oxidant, energy saving, and substrate universality. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
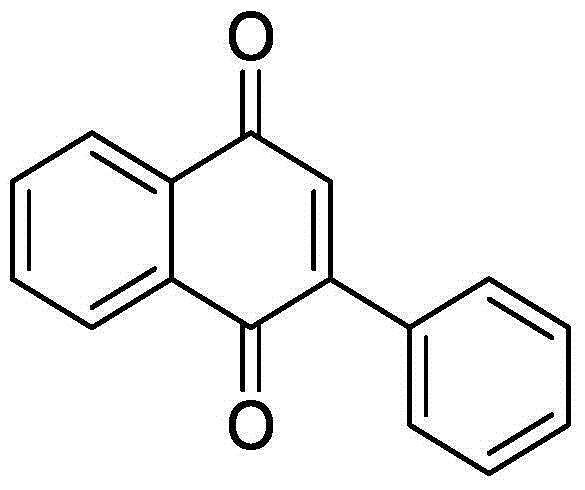
[0034] Add 44.0mg (0.2mmol) o-phenylethynyl acetophenone, 1.28mg (0.02mmol) Cu powder, 141.7mg (0.4mmol) Selectfluor, 19.6mg (0.2mmol) potassium acetate into a 10mL round bottom flask, and then add 2mL of acetonitrile / water (V:V=1:1) was used as solvent. Then, magnetically stir at 70° C. for 12 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-phenyl-1,4-naphthoquinone. The material was a yellow solid, 83% yield.
[0035] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ8.20-8.18(m, 1H), 8.13-8.12(m, 1H), 7.60-7.58(m, 2H), 7.50-7.48(m, 3H), 7.09(s, 1H);13 C NMR (CDCl 3 , 125MHz): δ185.0, 184.3, 148.1, 135.2, 133.8, 133.8, 133.4, 132.5, 132.1, 130.0, 129.4, 128.4, 127.0, 125.9.
Embodiment 2
[0037]
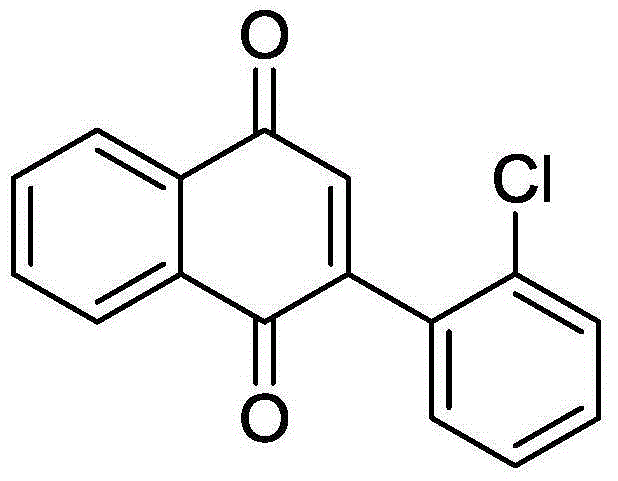
[0038] Add 50.9 mg (0.2 mmol) 2-o-chlorophenylethynylacetophenone, 3.98 mg (0.04 mmol) CuCl, 70.58 mg (0.2 mmol) Selectfluor, 27.6 mg (0.2 mmol) potassium carbonate to a 10 mL round bottom flask , and then added 2 mL of acetonitrile / water (V:V=2:1) as a solvent. Then, magnetic stirring was performed at 70° C. for 24 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-(2-chlorophenyl)-1,4-naphthoquinone. The material was a yellow solid in 85% yield.
[0039] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ=8.20-8.16(m, 2H), 7.82-7.81(m, 2H), 7.52(dd, J1=7.5Hz, J2=1Hz, 1H), 7.44-7.38(m, 2H), 7.31 (dd, J1=7.5Hz, J2=2Hz, 1H), 7.02(s, 1H); 13 C NMR (CDCl 3 , 125MHz): δ184.9, 183.1, 148.2, 137.4, 134.0, 133.9, 133.2,...
Embodiment 3
[0041]
[0042] Add 46.8 mg (0.2 mmol) 2-o-methylphenylethynylacetophenone, 1.28 mg (0.02 mmol) Cu powder, 141.7 mg (0.4 mmol) Selectfluor, 4.2 mg (0.1 mmol) potassium formate to a 10 mL round bottom In the flask, 2 mL of acetonitrile / water (V:V=5:1) was added as a solvent. Then, magnetically stir at 70° C. for 12 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-(2-methylphenyl)-1,4-naphthoquinone. The material was a yellow solid, 63% yield.
[0043] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ8.19-8.15(m, 2H), 7.81-7.79(m, 2H), 7.39-7.36(m, 1H), 7.32-7.27(m, 2H), 7.20(dd, J1=7.5Hz , J2=1Hz, 1H), 6.95(s, 1H), 2.25(s, 3H); 13 C NMR (CDCl 3 , 125MHz): δ185.2, 184.0, 150.7, 136.9, 136.2, 133.9, 133.9, 132.3, 132.2, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com