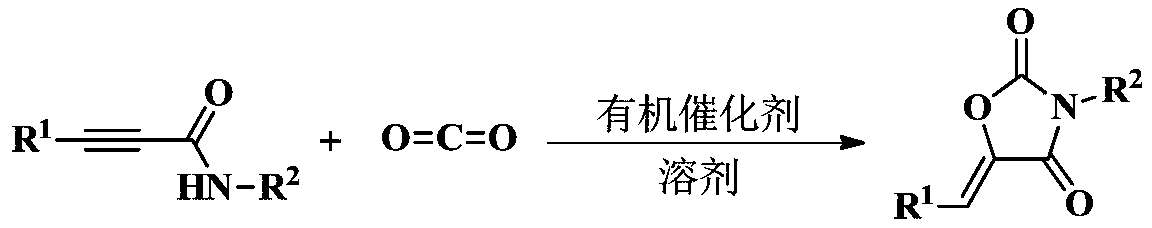
An organic amine catalyzed co 2 Method for synthesizing 2,4-oxazolidinediones
An oxazolidinedione, organic catalyst technology, applied in the direction of organic chemistry and the like, to achieve the effects of high target product yield, safe and simple reaction operation, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
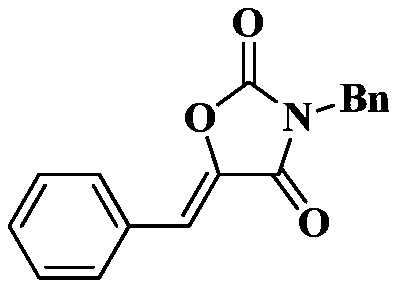
[0020] Under a carbon dioxide atmosphere, add a stirring bar, 0.5 mmol of N-benzyl-3-phenylpropynamide, 0.025 mmol of TBD, and 0.2 ml of tetrahydrofuran into a 10 ml Schlink bottle, and stir at 25 degrees Celsius for 1 hour, The reaction solution in the Schlinker flask was dissolved in 2 mL of dichloromethane and transferred to a 50 mL round-bottom single-necked flask. The Schlinker flask was rinsed with (3 x 2 mL) of methylene chloride, and the solvent was removed in vacuo to obtain the crude product . The crude product was separated and purified by column chromatography (eluent: dichloromethane). The yield was 98%.
[0021] The structural characterization data of the resulting product are as follows:
[0022]
[0023] 1 H NMR (400MHz, CDCl 3 )δ7.75(dd,J=7.5,2.1Hz,2H),7.49–7.40(m,5H),7.39–7.30(m,3H),6.78(s,1H),4.80(s,2H); 13 C NMR (126MHz, CDCl 3 )δ 162.1,152.1,137.7,134.6,131.2,130.8,130.6,129.1,129.0,129.0,128.7,113.8,43.9; IR:1807,1740,1678,1626,1496,1451,1437,140...
Embodiment 2
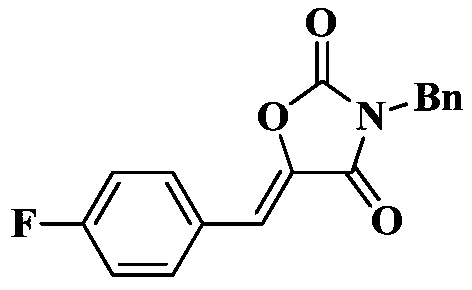
[0025] Under a carbon dioxide atmosphere, add a stir bar, 0.5 mmol of N-benzyl-3-(4-fluorophenyl) propynamide, 0.025 mmol of TBD, and 0.2 mL of tetrahydrofuran to a 10 mL Schlinker flask at 25 °C After stirring for 1 hour, the reaction solution in the Schlinker flask was dissolved in 2 mL of dichloromethane and transferred to a 50 mL round-bottomed single-necked flask. The crude product was obtained after solvent. The crude product was separated and purified by column chromatography (eluent: dichloromethane). The yield was 99%.
[0026] The structural characterization data of the resulting product are as follows:
[0027]
[0028] 1 H NMR (400MHz, CDCl 3 )δ7.73(dd,J=8.1,5.7Hz,2H),7.44(d,J=7.4Hz,2H),7.40–7.27(m,3H),7.10(t,J=8.4Hz,2H), 6.72(s,1H),4.78(s,2H). 13 C NMR (101MHz, CDCl 3 )δ165.0, 162.2(d, J=54.1Hz), 151.9, 137.2(d, J=2.7Hz), 134.4, 133.2(d, J=8.6Hz), 129.0, 129.0, 128.6, 127.0(d, J=3.4 Hz),116.3(d,J=21.9Hz),112.5,43.9.IR:1809,1736,1671,1439,1407,1348,1241,...
Embodiment 3
[0030] Under a carbon dioxide atmosphere, add a stirring bar, 0.5 mmol of N-benzyl-3-(p-tolyl) propynamide, 0.025 mmol of TBD, and 0.2 ml of tetrahydrofuran to a 10 ml Schlink flask, and stir at 25 degrees Celsius for 1 After 2 hours, the reaction solution in the Schlinker flask was dissolved in 2 mL of dichloromethane and transferred to a 50-ml round-bottomed single-necked flask. The Schlinker flask was rinsed with (3 × 2 mL) methylene chloride, and the solvent was removed in vacuo. A crude product is obtained. The crude product was separated and purified by column chromatography (eluent: dichloromethane). The yield was 83%.
[0031] The structural characterization data of the resulting product are as follows:
[0032]
[0033] 1 H NMR (500MHz, CDCl 3 )δ7.62(d, J=8.1Hz, 2H), 7.44(d, J=6.7Hz, 2H), 7.39–7.28(m, 3H), 7.21(d, J=8.0Hz, 2H), 6.74( s,1H),4.77(s,2H),2.37(s,3H). 13 C NMR (126MHz, CDCl 3)δ162.2, 152.2, 141.3, 137.1, 134.6, 131.3, 129.9, 129.0, 129.0, 128.6, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com