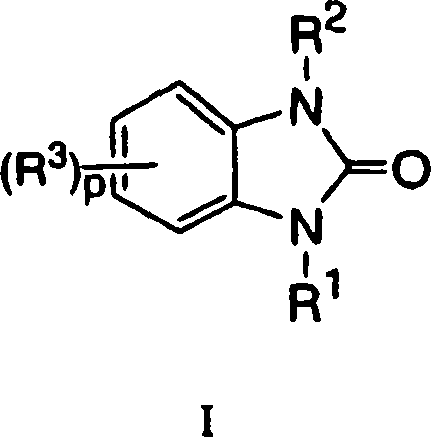
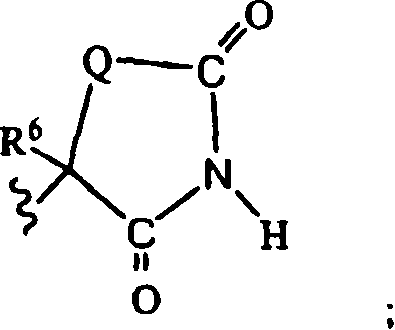
Benzoureas having anti-diabetic activity
A compound, phenyl technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, diseases, etc., can solve the problems of negative effects of lipids, inability to significantly improve lipid metabolism, weak efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0153] The following reaction scheme examples are provided to illustrate the invention and should not be construed as limiting the invention in any way. The scope of the invention is defined by the claims.
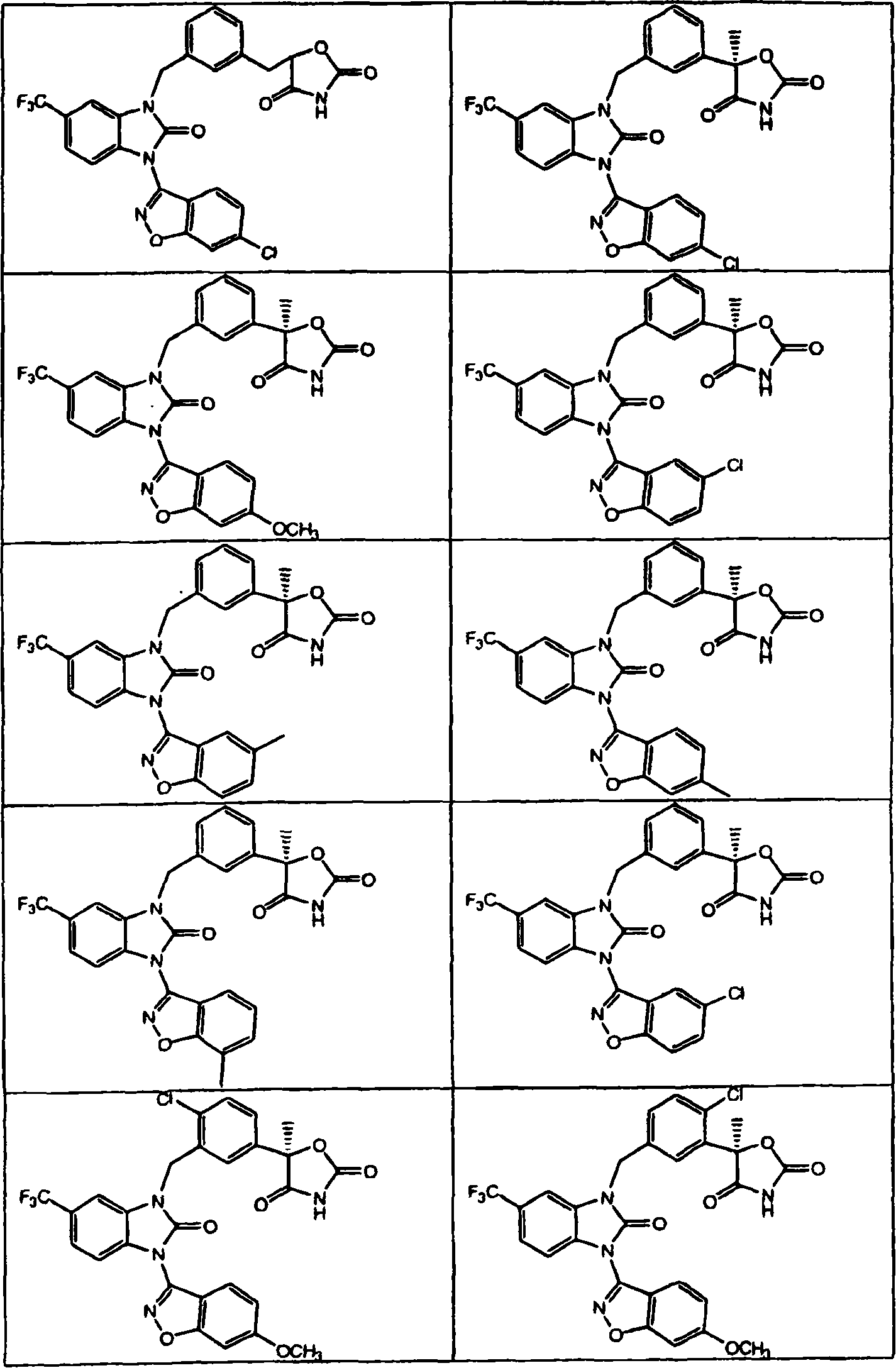
[0154] The formulas in Table 1 further illustrate compounds that are made or can be made using the methods disclosed herein.
[0155] The synthesized compounds in Table 1 were analyzed by high pressure liquid chromatography-mass spectrometry (LC-MS) and / or proton NMR. LC-MS samples were analyzed using an Agilent 1100 Series High Pressure Liquid Chromatography coupled to a Waters MicromassZQ mass spectrometer. The column used was Waters Xterra and the compound was eluted using a gradient elution program (10% B to 100% B in 4.5 min) at a flow rate of 2.5 mL / min. Solvent A: water containing 0.06% trifluoroacetic acid. Solvent B: Acetonitrile containing 0.05% trifluoroacetic acid. Retention times are given in minutes.
[0156] Method A: XTerra MS-C18, 4.5 x 50mm, 10-100% ...
Embodiment 1
[0248]
[0249] (5R)-5-(3-{[3-(5-chloro-1,2-benzisoxazol-3-yl)-2-oxo-2,3-dihydro-1H-benzimidazole -1-yl]methyl}phenyl)-5-methyl-1,3-oxazolidine-2,4-dione:
[0250] 1 H NMR (500MHz, CDCl 3 )δ8.39(s, 1H), 8.33(brs, 1H), 7.92(d, 1H), 7.71(s, 1H), 7.61(s, 2H), 7.58(d, 1H), 7.42(m, 2H ), 7.21(d, 2H), 7.02(d, 1H), 5.23(dd, 2H), 1.99(s, 3H).
Embodiment 2
[0252]
[0253] (5R)-5-(4-chloro-3-{[3-(6-methoxy-1,2-benzisoxazol-3-yl)-2-oxo-6-(trifluoromethane Base)-2,3-dihydro-1H-benzimidazol-1-yl]methyl}phenyl)-5-methyl-1,3-oxazolidine-2,4-dione:
[0254] 1 H NMR (500MHz, CDCl 3 )δ8.14(d, 1H), 8.02(d, 1H), 7.67(s, 1H), 7.57(s, 1H), 7.52(d, 1H), 7.26(m, 2H), 7.09(s, 1H ), 7.06(d, 1H), 5.38(s, 2H), 3.98(s, 3H), 1.83(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com