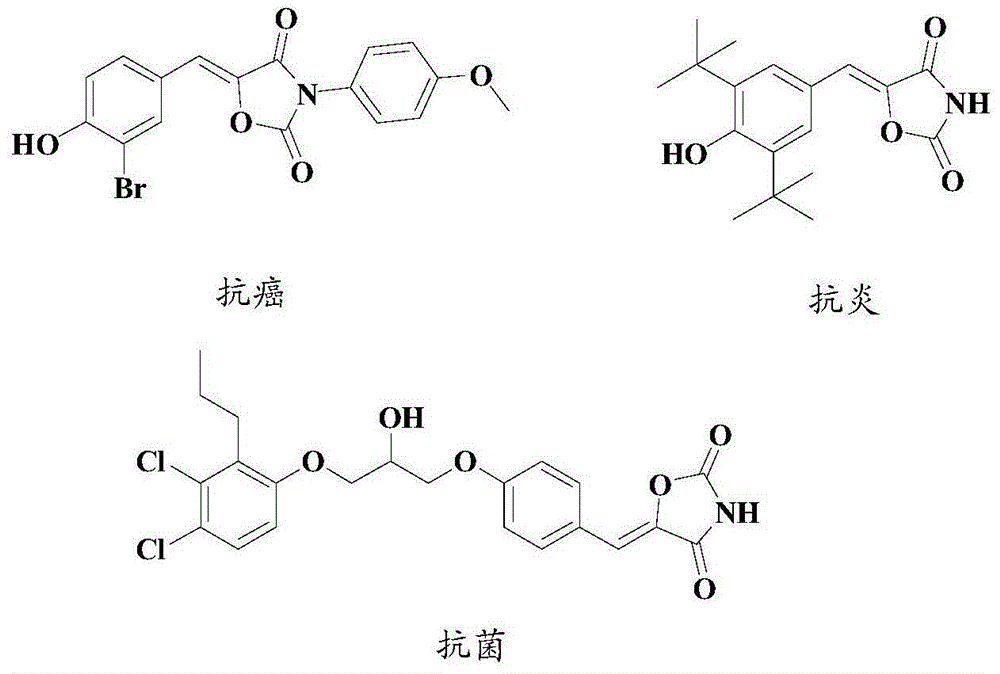
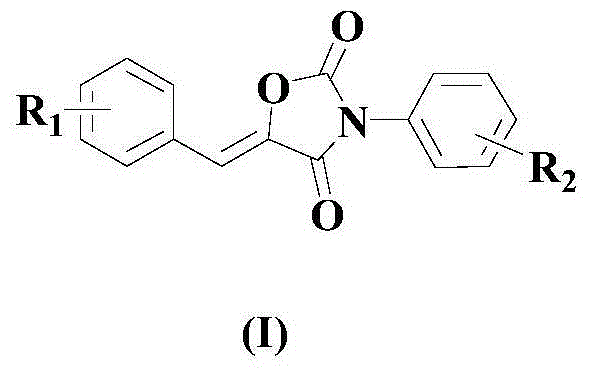
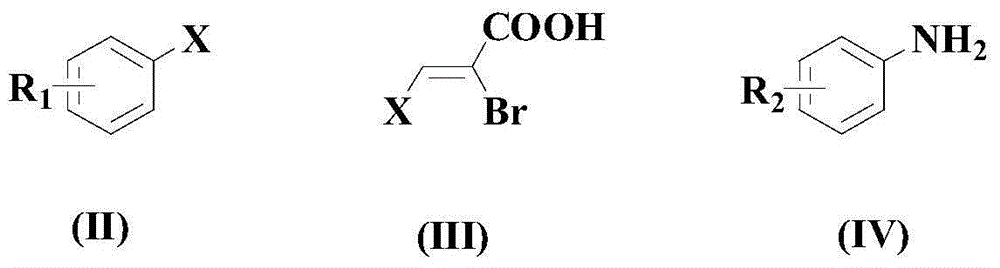Synthesis method of drug intermediate oxazolidinedione compound
A technology of oxazolidinedione and synthetic method, which is applied in the direction of organic chemistry, can solve the problems that cannot be well utilized, the methods are reported, and the environment, and achieve the effect of broad application prospects and industrialization potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Add 100mmol formula (II) compound, 150mmol formula (III) compound and 200mmol formula (IV) compound, carbon dioxide gas Purge several times until it becomes a carbon dioxide atmosphere; add 3mm ol PdCl under stirring 2(dppe), 200mmol DABCO and 5mmol additives (a mixture of 2mmol 2-fluorophenylboronic acid pinacol ester and 3mmol CTAB); continue to feed carbon dioxide gas into the reaction system, and raise the temperature to 60°C, at this temperature The reaction was stirred for 9 hours.
[0041] After the reaction, cool down to room temperature naturally, then add a sufficient amount of saturated aqueous sodium bicarbonate solution to the reaction system, fully oscillate, mix, stand and separate the layers, separate the organic phase, wash with water again, and separate the organic phase again Dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and recrystallize the residue with isopropanol to obtain the target compound of...
Embodiment 2
[0045]
[0046] Add 100mmol formula (II) compound, 165mmol formula (III) compound and 250mmol formula (IV) compound, carbon dioxide gas Purge several times until it becomes a carbon dioxide atmosphere; add 5mmol PdCl under stirring 2 (dppe), 250mmol DABCO and 7mmol additives (a mixture of 3mmol 2-fluorophenylboronic acid pinacol ester and 4mmol CTAB); continue to feed carbon dioxide gas into the reaction system, and raise the temperature to 70°C, at this temperature The reaction was stirred for 8 hours.
[0047] After the reaction, cool down to room temperature naturally, then add a sufficient amount of saturated aqueous sodium bicarbonate solution to the reaction system, fully oscillate, mix, stand and separate the layers, separate the organic phase, wash with water again, and separate the organic phase again Dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and recrystallize the residue with isopropanol to obtain the target compound of...
Embodiment 3
[0051]
[0052] Add 100mmol formula (II) compound, 180mmol formula (III) compound and 225mmol formula (IV) compound, carbon dioxide gas Purge several times until it becomes a carbon dioxide atmosphere; add 6mm ol PdCl under stirring 2 (dppe), 300mmol DABCO and 8mmol additives (a mixture of 3.6mmol 2-fluorophenylboronic acid pinacol ester and 4.4mmol CTAB); continue to feed carbon dioxide gas into the reaction system, and raise the temperature to 90°C. The reaction was stirred at temperature for 6 hours.
[0053] After the reaction, cool down to room temperature naturally, then add a sufficient amount of saturated aqueous sodium bicarbonate solution to the reaction system, fully oscillate, mix, stand and separate the layers, separate the organic phase, wash with water again, and separate the organic phase again Dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and recrystallize the residue with isopropanol to obtain the target compound of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com