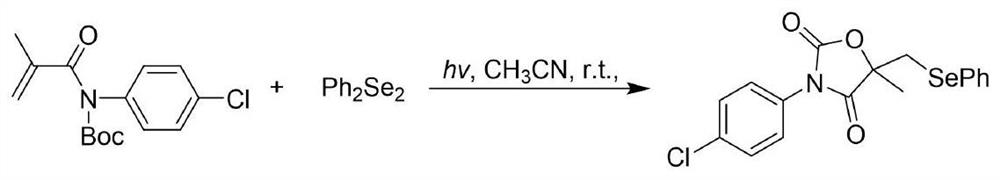A visible light-promoted synthesis method of selenooxazolidine-2,4-dione
A technology of selenooxazolidine and synthetic method, which is applied in the direction of organic chemistry, can solve problems that have not been reported, and achieve the effects of good application potential, high reaction efficiency, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
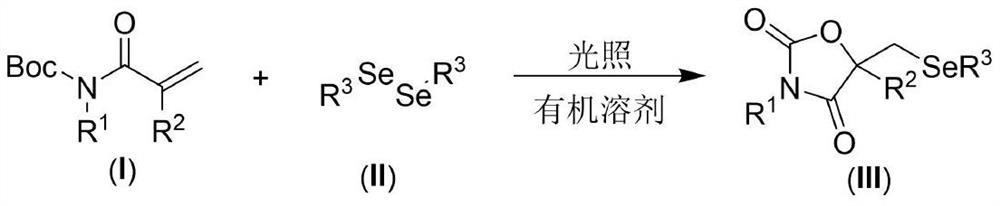
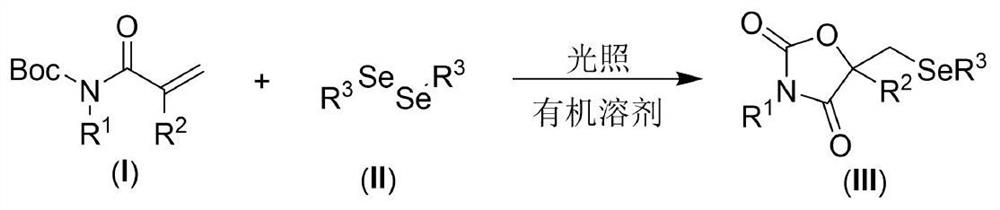
[0021] The present invention provides the following technical scheme: a visible light-promoted synthetic method of selenooxazolidine-2.4-dione, the steps are as follows: in an organic solvent, with N-Boc-protected propylene having the structure shown in formula (I) Amide, the diselenide of the structure shown in formula (II) is the reaction raw material, reacts under the irradiation of visible light, after the reaction is finished, the reaction solution is decompressed to remove the solvent to obtain the crude product, and the crude product is purified by column chromatography to obtain the formula (III ) a selenooxazolidine-2.4-dione compound of the structure shown in );
[0022] The reaction equation is shown in the following formula:
[0023]
[0024] wherein R in the compound of formula (I) 1 and R 2 is an H atom, an alkyl group and an aryl group, and the compound of formula (II) adopts diaryl diselenide and dialkyl diselenide.
Embodiment 1
[0026] The reaction equation is shown in the following formula:
[0027]
[0028] At room temperature, N-Boc-N-(4-chlorophenyl)-2-methacrylamide (0.25 mmol), diphenyl diselenide, (0.25 millimoles), acetonitrile (2 milliliters), after adding, a 23-watt white compact fluorescent lamp was placed 3 centimeters away from the reaction tube, and reacted at room temperature for 36 hours. After the reaction was completed, the organic phase was removed by a rotary evaporator The solvent and the residue were purified with a silica gel column (silica gel specifications: 200-300 mesh, eluent petroleum ether / ethyl acetate=20:1) to obtain 93 mg of a white solid with a yield of 94%.
[0029] The obtained product nuclear magnetic spectrum data are: 11 H NMR (400MHz, CDCl 3 ): δ7.56-7.52 (m, 2H), 7.46 (d, J=8.8Hz, 2H), 7.39 (d, J=8.8Hz, 2H), 7.30-7.25 (m, 3H), 3.46 (d, J=13.9Hz, 1H), 3.41(d, J=13.9Hz, 1H), 1.77(s, 3H). 13 C NMR (100MHz, CDCl 3 ): δ173.2, 152.8, 134.8, 133.6, 129.5, 129....
Embodiment 2
[0031] The reaction equation is shown in the following formula:
[0032]
[0033] At room temperature, N-Boc-N-(4-bromophenyl)-2-methacrylamide (0.25 mmol), diphenyl diselenide, (0.25 millimoles), acetonitrile (2 milliliters), after adding, a 23-watt white compact fluorescent lamp was placed 3 centimeters away from the reaction tube, and reacted at room temperature for 36 hours. After the reaction was completed, the organic phase was removed by a rotary evaporator The solvent and the residue were purified with a silica gel column (silica gel specifications: 200-300 mesh, eluent: petroleum ether / ethyl acetate = 25:1) to obtain 104 mg of white solid with a yield of 95%.
[0034] The obtained product nuclear magnetic spectrum data are: 1 H NMR (400MHz, CDCl 3 ):δ7.54(d,J=8.8Hz,2H),7.49–7.42(m,2H),7.25(d,J=8.8Hz,2H),7.23–7.16(m,3H),3.38(d, J=13.9Hz,1H),3.33(d,J=13.9Hz,1H), 1.69(s,3H). 13 C NMR (100MHz, CDCl 3): δ172.1, 151.7, 132.5, 131.5, 128.9, 128.4, 127.6, 127.1, 126.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com