Preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione
A technology of oxazolidinedione and benzyloxycarbonyl, which is applied in the field of chemical synthesis and production, and can solve the problems of large influence on yield, easy ring opening and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
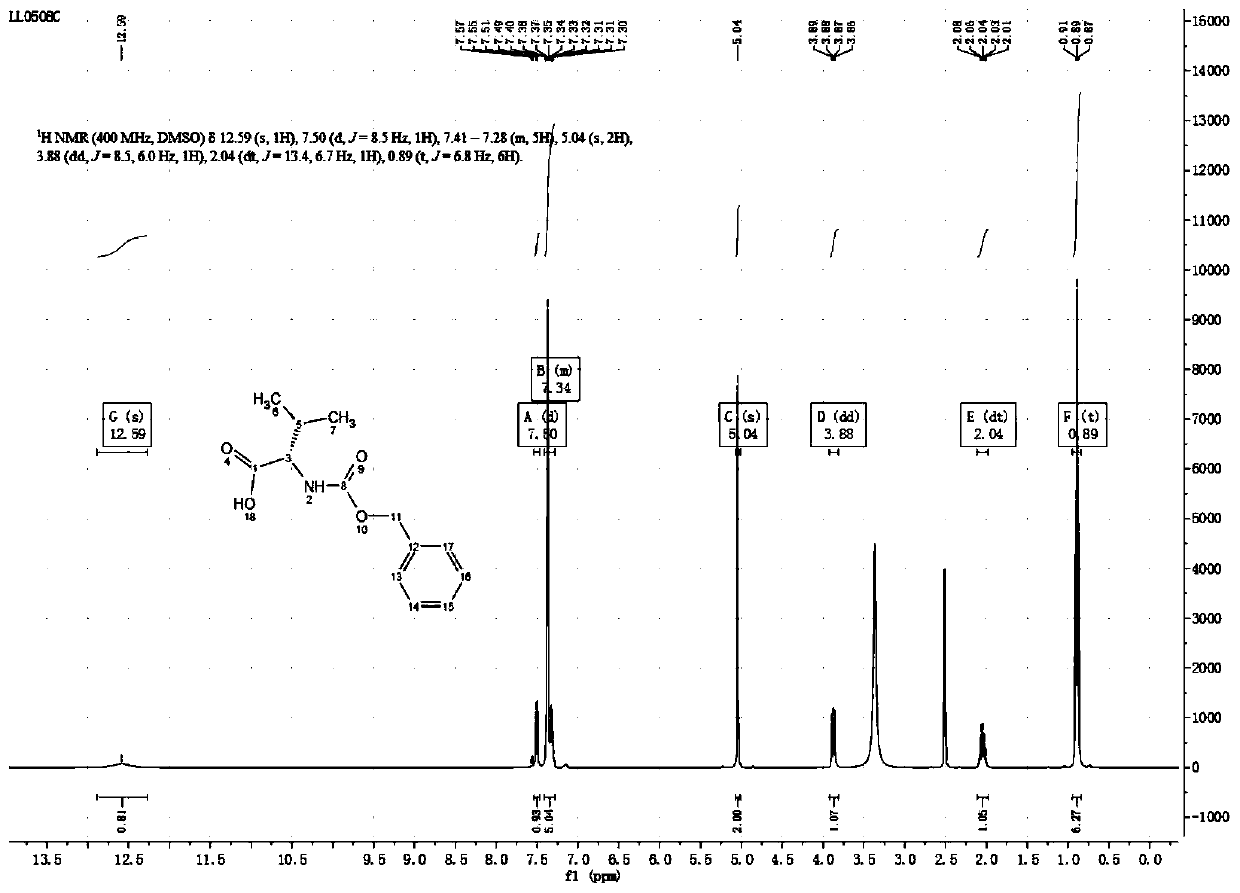
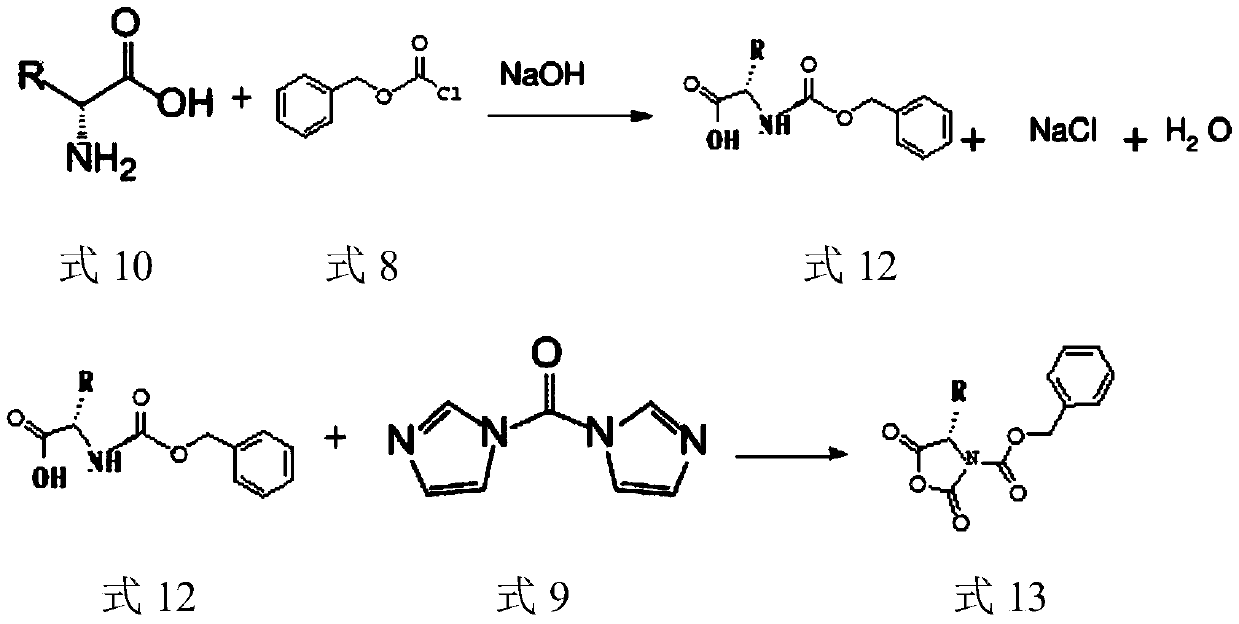
[0049] The synthesis of embodiment 1 N-benzyloxycarbonyl-L-valine
[0050] Get 1.17Kg (10mol) of L-valine, 5L of sodium hydroxide solution of 2mol / L, 1.06Kg (10mol) sodium carbonate and add in the 20L reactor, start stirring, after the L-valine dissolves completely, dissolve the solution When the temperature dropped below 0°C, 5L of 1,4-dioxane solution containing 2.05Kg (12mol) of benzyl chloroformate was added dropwise, and the temperature of the solution was kept below 20°C during the dropping process. After the reaction, the reaction solution was extracted with 2.5L of dichloromethane, the organic phase was discarded, the water phase was cooled to below 10°C, concentrated hydrochloric acid was added dropwise until the pH = 2, and then stirred at 10°C for 30min, a large amount of white solid was precipitated, and filtered with suction , the filter residue was washed with water, and the white solid was dried in a vacuum oven to obtain 2.35Kg of white N-benzyloxycarbonyl-L-va...
Embodiment 2
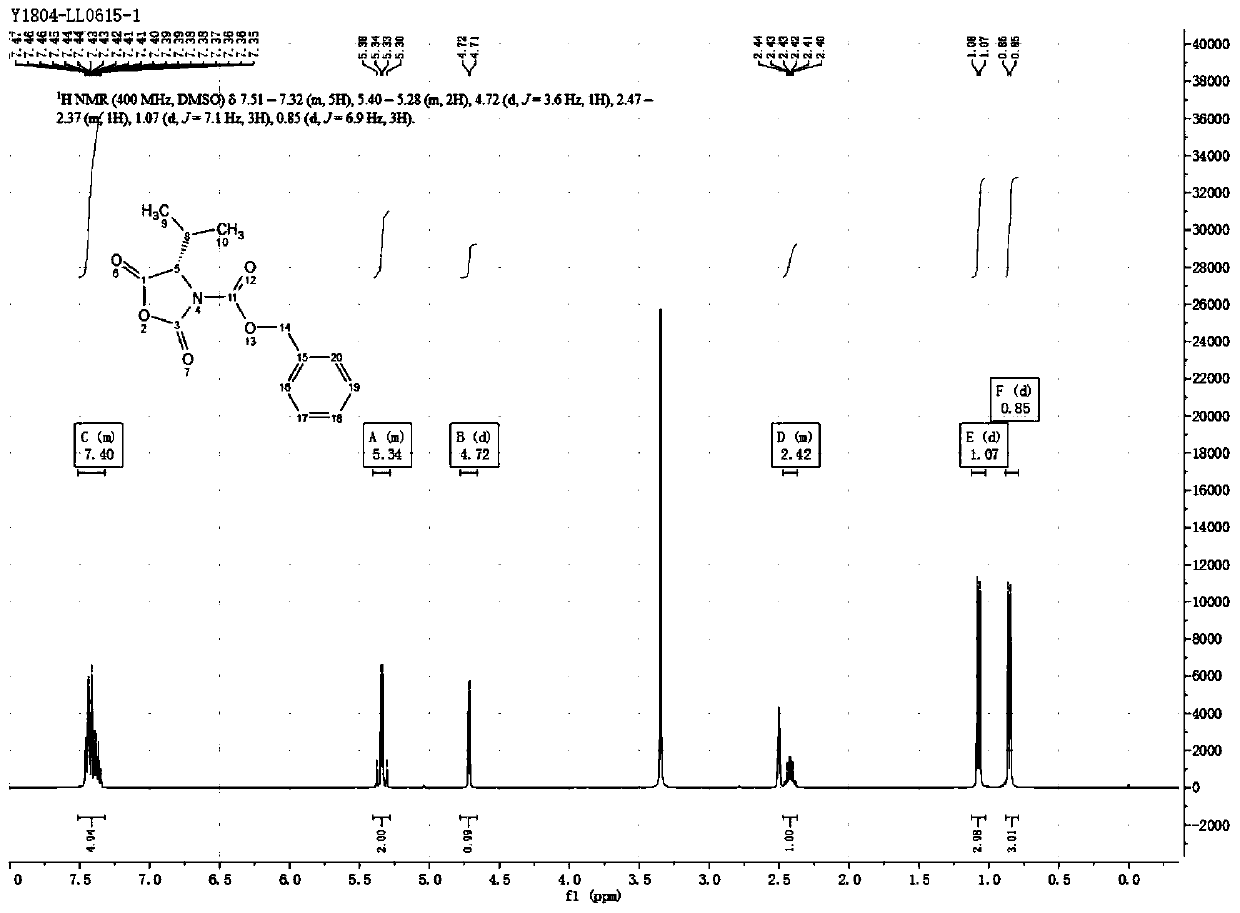
[0052] The synthesis of embodiment 2 (S)-3-benzyloxycarbonyl-4-isopropyl-2,5-oxazolidinedione
[0053] Get 1.17Kg (10mol) of L-valine, 5L of sodium hydroxide solution of 2mol / L, 1.06Kg (10mol) sodium carbonate and add in the 20L reactor, start stirring, after the L-valine dissolves completely, dissolve the solution When the temperature dropped below 0°C, 5L of 1,4-dioxane solution containing 2.05Kg (12mol) of benzyl chloroformate was added dropwise, and the temperature of the solution was kept below 20°C during the dropping process. After the reaction is complete, the reaction solution is extracted with 2.5 L of dichloromethane, the organic phase is discarded, the water phase is cooled to below 10°C, concentrated hydrochloric acid is added dropwise until pH=2, the mixture is extracted 3 times with 3 L of dichloromethane, and the organic phases are combined. The organic phase was washed with 3L saturated brine, dried over anhydrous sodium sulfate for 12 hours, filtered, and the...
Embodiment 3
[0054] The synthesis of embodiment 3 (S)-3-benzyloxycarbonyl-4-isopropyl-2,5-oxazolidinedione
[0055] Get 1.17Kg (10mol) of L-valine, 5L of sodium hydroxide solution of 2mol / L, 1.06Kg (10mol) sodium carbonate and add in the 20L reactor, start stirring, after the L-valine dissolves completely, dissolve the solution When the temperature dropped below 0°C, 5L of 1,4-dioxane solution containing 2.05Kg (12mol) of benzyl chloroformate was added dropwise, and the temperature of the solution was kept below 20°C during the dropping process. After the reaction is complete, the reaction solution is extracted with 2.5 L of dichloromethane, the organic phase is discarded, the water phase is cooled to below 10°C, concentrated hydrochloric acid is added dropwise until pH=2, the mixture is extracted 3 times with 3 L of dichloromethane, and the organic phases are combined. The organic phase was washed with 3L saturated brine, dried over anhydrous sodium sulfate for 12 hours, filtered, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com