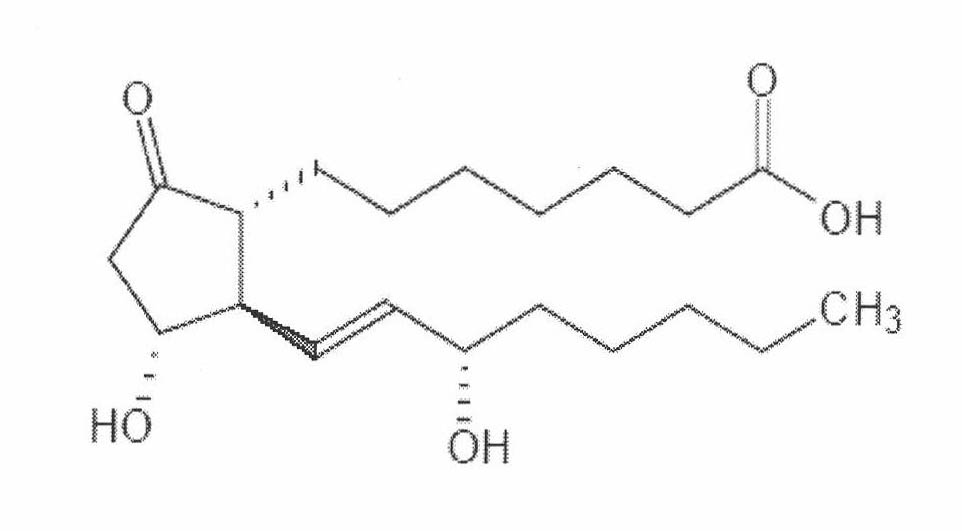
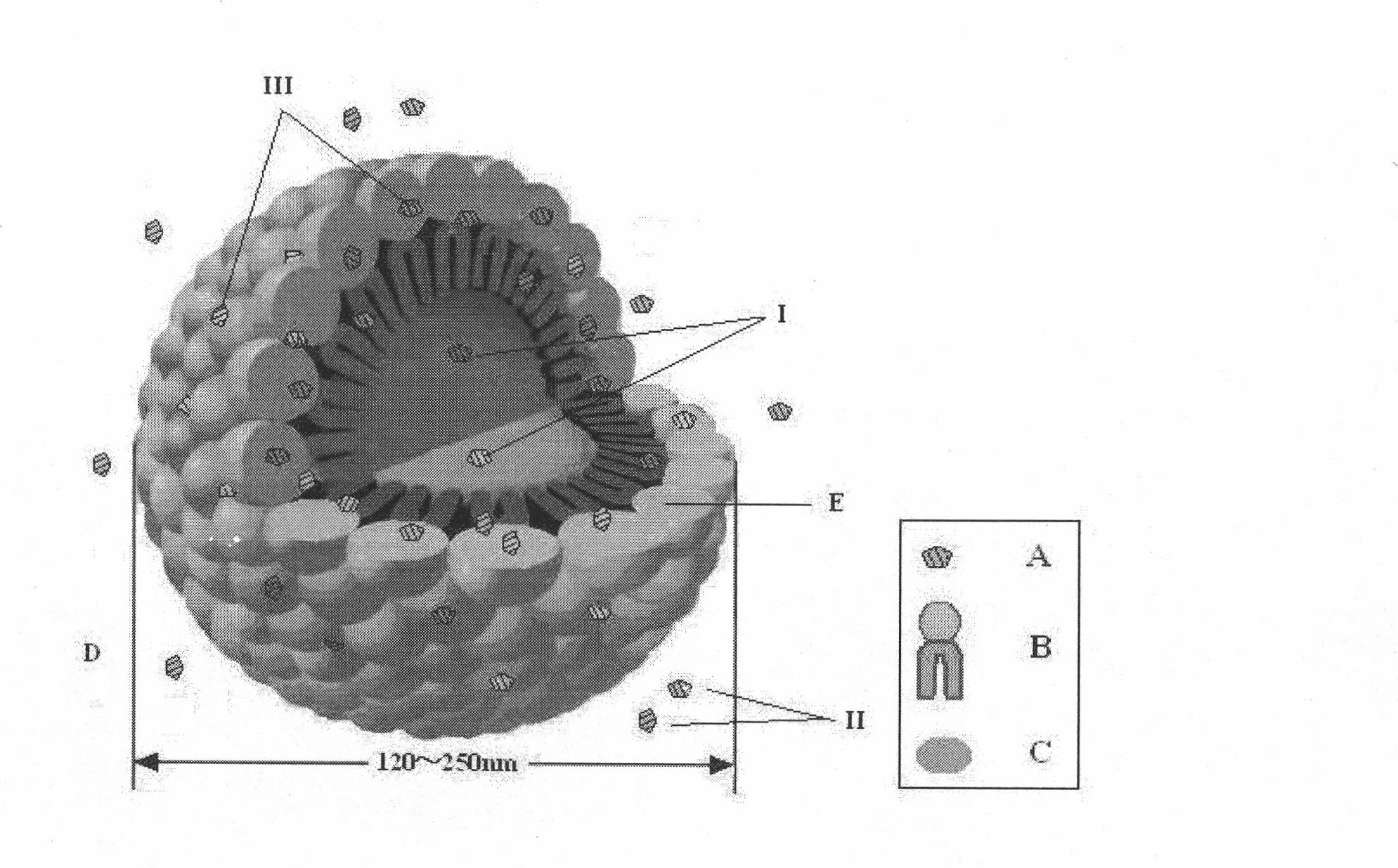
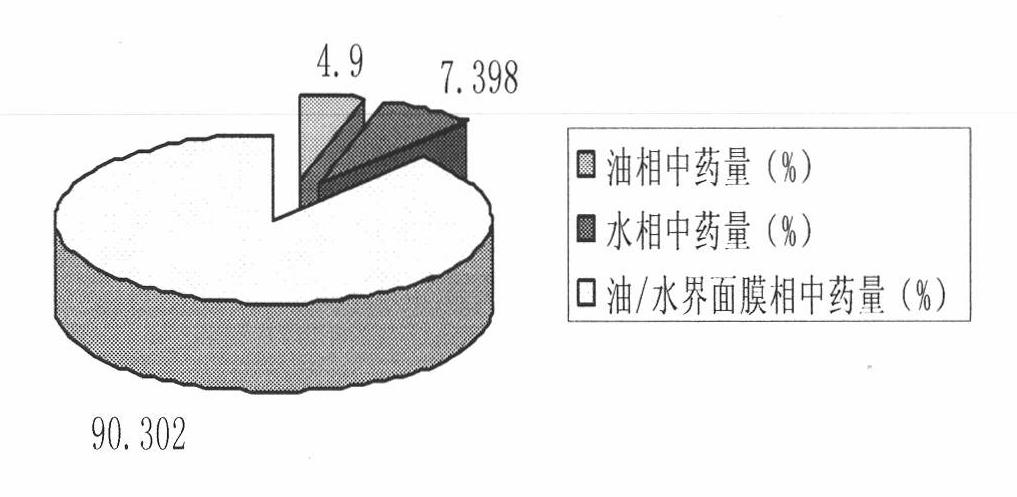
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
A technology of prostaglandins and lipid microspheres, which is applied in the field of medicine, can solve the problems of not giving full play to the advantages of lipid microspheres, and achieve the effects of improving the body's tolerance, solving large doses, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Prescription: prostaglandin 0.5mg, egg yolk lecithin 1.5g, medium chain oil for injection 8g, sodium hydroxide appropriate amount, glycerin 2.5g, the rest is water for injection, 100ml in total.
[0072] Process:
[0073] (1) Preparation of oil phase: Add medium chain oil for injection into the preparation tank, control the temperature of medium chain oil for injection at 40°C, add egg yolk lecithin, stir vigorously until dissolved, cool down to 30°C, add medicine, stir to mix uniform;
[0074] (2) Preparation of water phase: Stir water and glycerin at 40°C for 5 minutes to make them completely miscible;
[0075] (3) At 30°C, add the oil phase to the water phase and stir vigorously to form colostrum;
[0076] (4) adjust the pH value to 5 with sodium hydroxide;
[0077] (5) Homogenizer: adjust the homogenization pressure to 500bar in the first step, and then adjust it to 1000bar in the second step, and homogenize the solution repeatedly;
[0078] (6) The emulsion obt...
Embodiment 2
[0080] Prescription: prostaglandin 0.5mg, soybean lecithin 1.5g, soybean oil for injection 10g, glucose 5g, appropriate amount of sodium citrate, and the rest water for injection, 100ml in total.
[0081] Process:
[0082] (1) Preparation of oil phase: add soybean oil for injection into the preparation tank, control the temperature of soybean oil for injection at 60°C, add soybean lecithin, and stir vigorously until dissolved;
[0083] (2) Preparation of water phase: Stir water and glucose at 55°C for 5 minutes to make them completely miscible;
[0084] (3) At 60°C, add the oil phase to the water phase and stir vigorously to form colostrum;
[0085] (4) adjust the pH value to 5.8 with sodium hydroxide;
[0086] (5) Homogenizer: adjust the homogenization pressure to 600bar in the first step, and then adjust it to 1500bar in the second step, and homogenize the solution repeatedly;
[0087] (6) The emulsion obtained above is sterilized by filtration with a 0.22um microporous m...
Embodiment 3
[0089] Prescription: prostaglandin 0.5mg, egg yolk lecithin 1.2g, soybean oil for injection 6g, glyceryl caprylate 4g, mannitol 5g, oleic acid 0.01g, appropriate amount of sodium hydroxide, the rest is water for injection, a total of 100ml. (attached Figure 23 )
[0090] Process: Under nitrogen protection throughout the process
[0091] (1) Preparation of the oil phase: Add soybean oil for injection, glyceryl caprylate and oleic acid into the preparation tank, control the temperature at 60°C, add egg yolk lecithin, stir vigorously until dissolved, cool down to 50°C and add the drug, Mix vigorously;
[0092] (2) Preparation of water phase: Stir water and mannitol at 50°C for 5 minutes to make them completely miscible;
[0093] (3) At 30°C, add the water phase to the oil phase, stir vigorously to form colostrum;
[0094] (4) adjust the pH value to 6 with sodium hydroxide;
[0095] (5) Homogenizer: adjust the homogenization pressure to 400bar in the first step, and then adj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com