Atorvastatin calcium nano-lipid carrier and preparation method thereof
A technology of atorvastatin calcium and nano-lipid carrier, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, metabolic diseases, etc., can solve problems such as easy leakage and low encapsulation rate, and achieve low toxicity and improved Solubility, diameter reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Dissolve 10mg of atorvastatin calcium, 30mg of glyceryl monostearate, 20mg of corn oil, and 150mg of soybean lecithin in 5ml of ethanol, heat in a water bath at 65°C until completely dissolved, and form an organic phase; another 250mg of poloxa Mel 188 was dissolved in 15ml of water, and heated in a water bath to 65°C to form the water phase; under 1000r / min magnetic stirring, the organic phase was added dropwise to the water phase with a No. 5ml to form colostrum; quickly disperse the colostrum into 25ml of ice water stirred at 1000r / min, continue to stir and solidify in the ice bath for 1.5h, and prepare the atorvastatin calcium nano-lipid carrier.
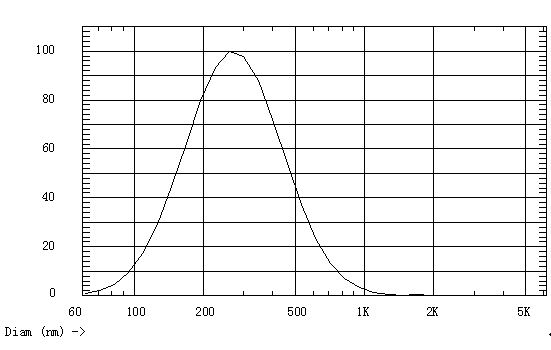
[0043] The prepared nano-lipid carrier is suitably diluted with distilled water, and the particle size and its distribution are measured. The particle size distribution is as follows: figure 1 As shown, the average particle diameter is 303.5nm, the PDI is 0.232, and the zeta potential is -13.84mV.
Embodiment 2
[0045] Dissolve 10mg of atorvastatin calcium, 20mg of glyceryl monostearate, 40mg of corn oil, and 140mg of soybean lecithin in 5ml of ethanol, heat in a water bath at 65°C until completely dissolved, and form an organic phase; another 100mg of poloxamer 188. Dissolve 100mg of Tween 80 in 15ml of water, heat in a water bath to 65°C to form the water phase; under magnetic stirring at 1000r / min, add the organic phase to the water phase drop by drop with a No. 7 needle, continue stirring, evaporate the organic phase and Concentrate the volume to 5ml to form colostrum; quickly disperse the colostrum into 25ml of ice water stirred at 1000r / min, continue to stir and solidify in the ice bath for 1.5h, and prepare the atorvastatin calcium nano-lipid carrier with an encapsulation efficiency of 82.1%.
Embodiment 3
[0050] Dissolve 5mg of atorvastatin calcium, 50mg of glyceryl monostearate, 20mg of corn oil, and 130mg of soybean lecithin in 5ml of ethanol, heat in a water bath at 65°C until completely dissolved, and form an organic phase; another 200mg of poloxamer Dissolve 188 in 15ml of water, heat in a water bath to 65°C to form the water phase; add the organic phase to the water phase drop by drop with a No. 7 needle under magnetic stirring at 1200r / min, continue stirring, evaporate the organic phase and concentrate the volume to 5ml , to form colostrum; quickly disperse the colostrum into 25ml of ice water stirred at 1000r / min, continue to stir and solidify under the ice bath for 1.5h, and prepare the atorvastatin calcium nano-lipid carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com