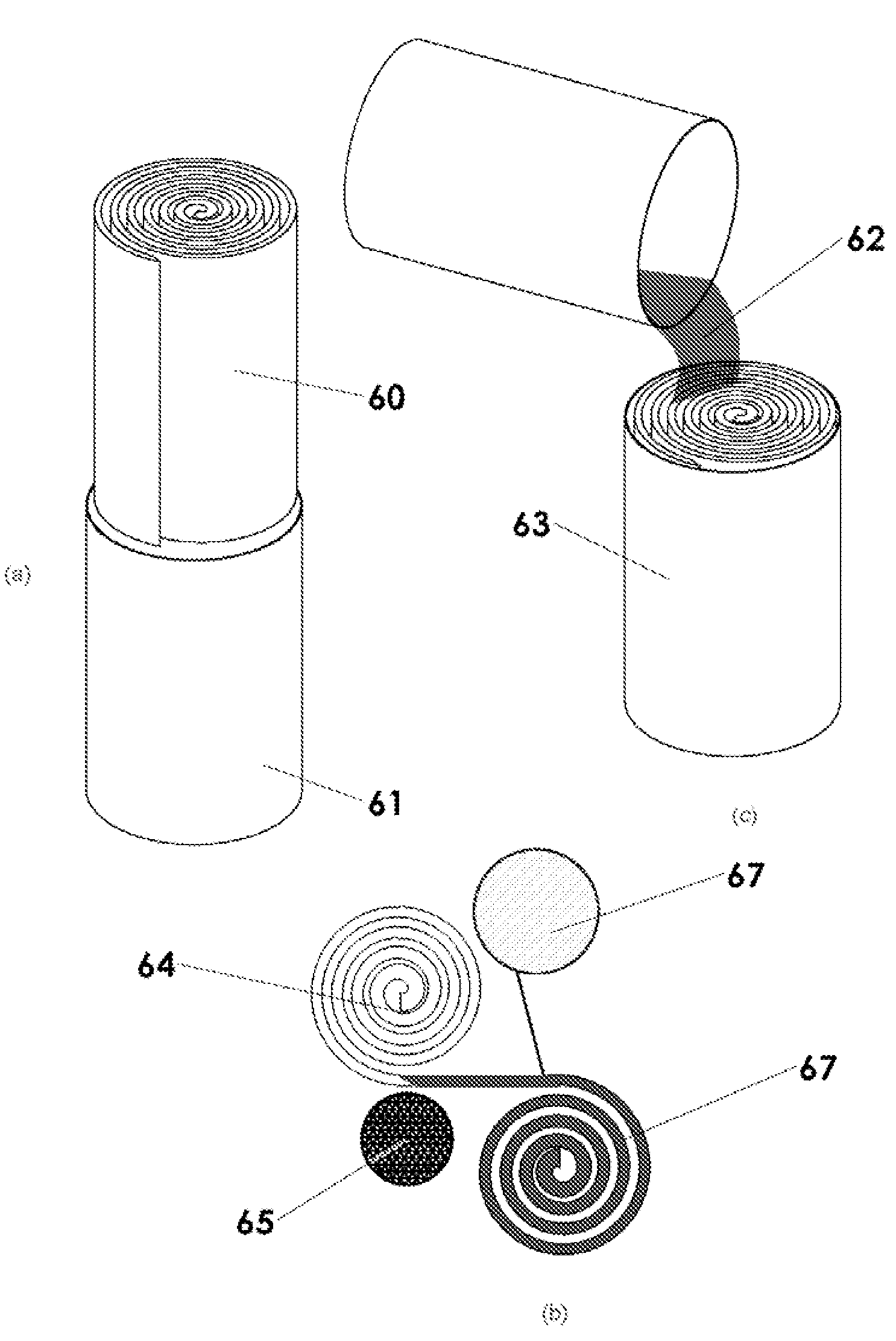
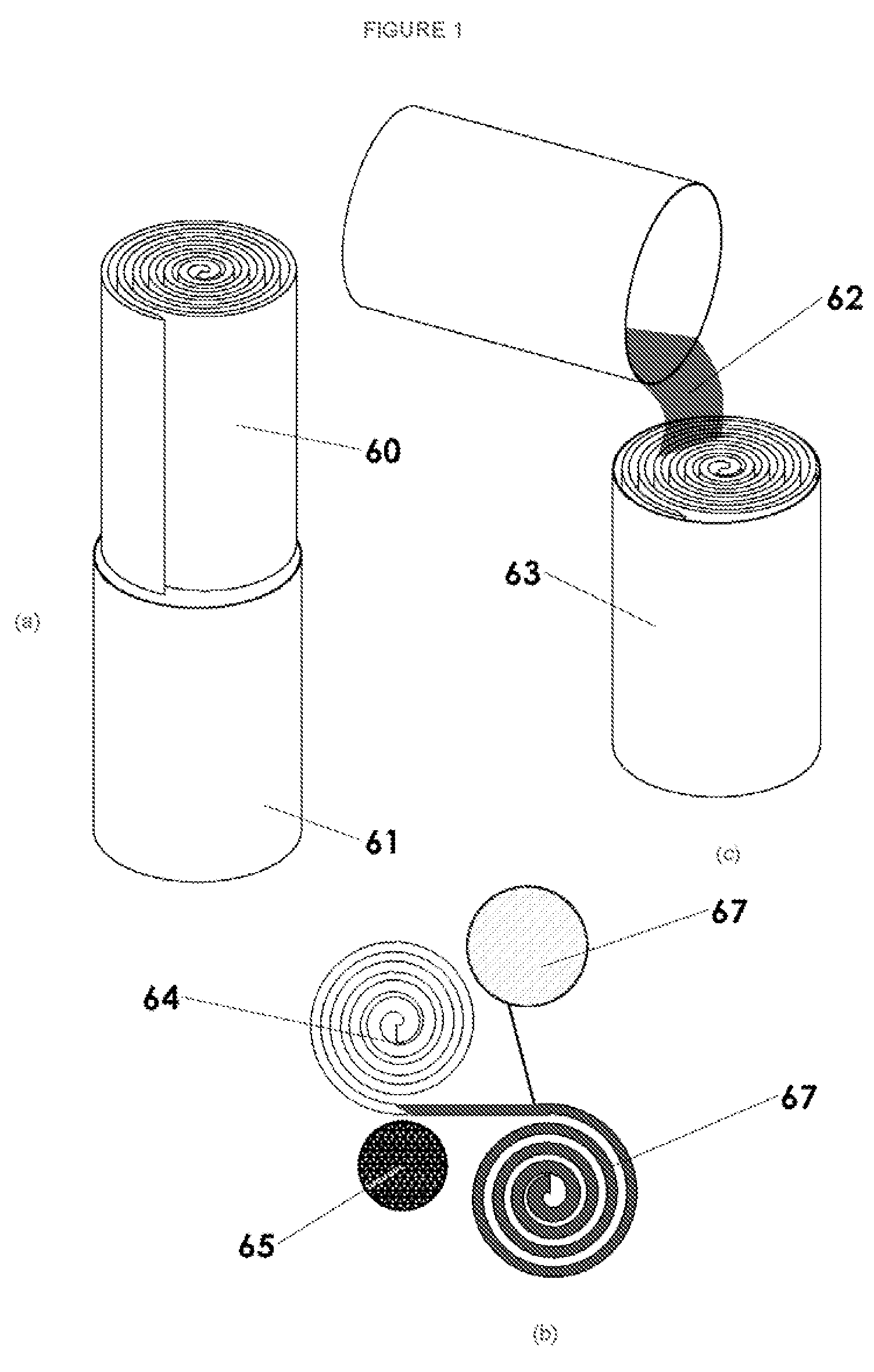
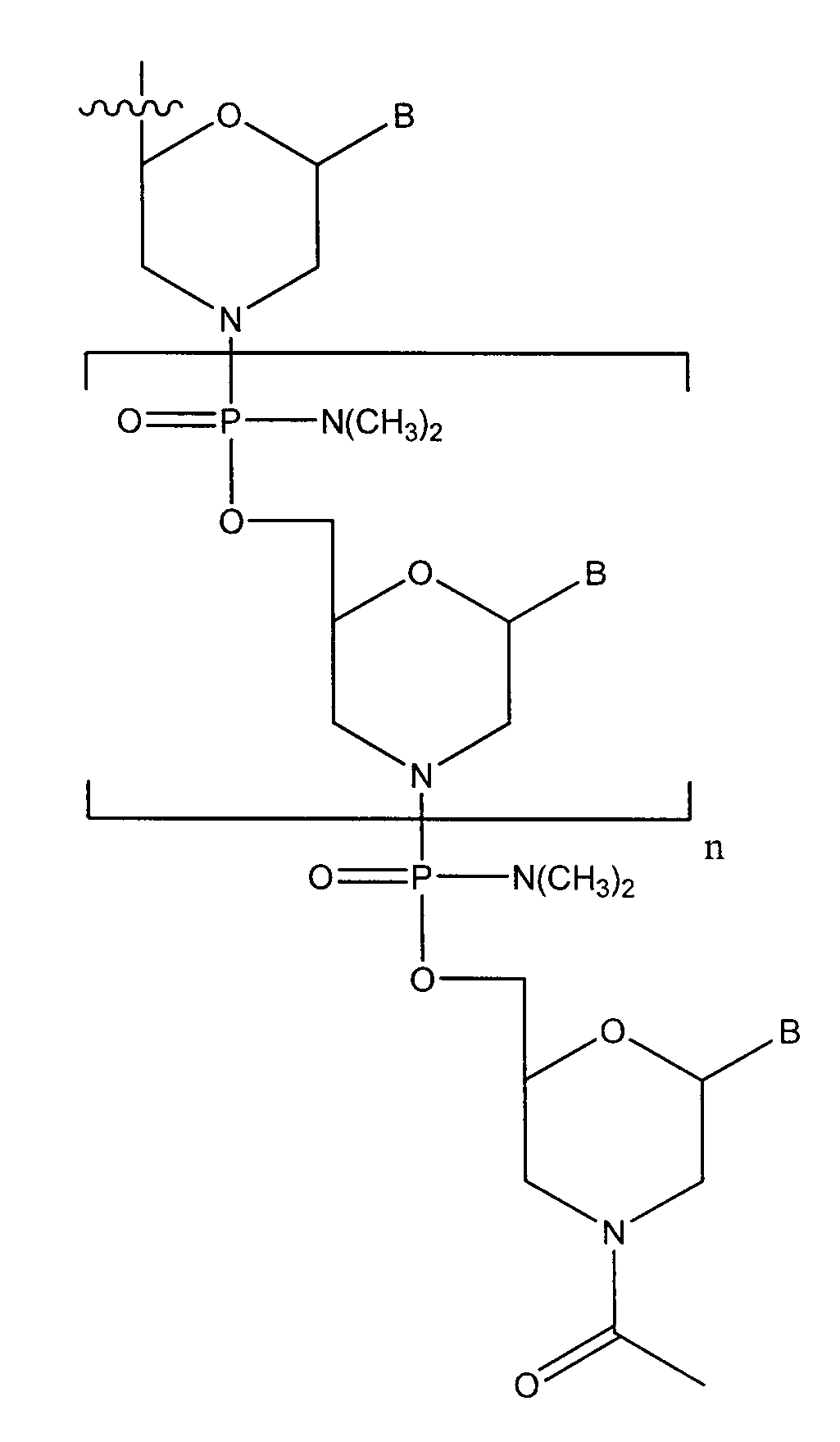
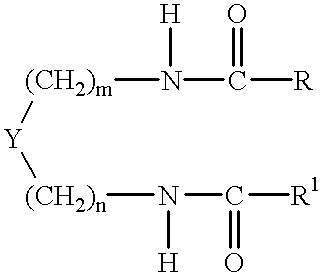
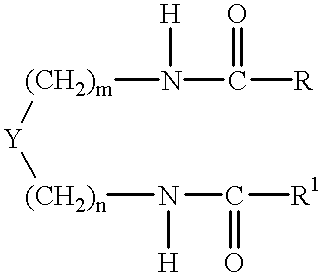
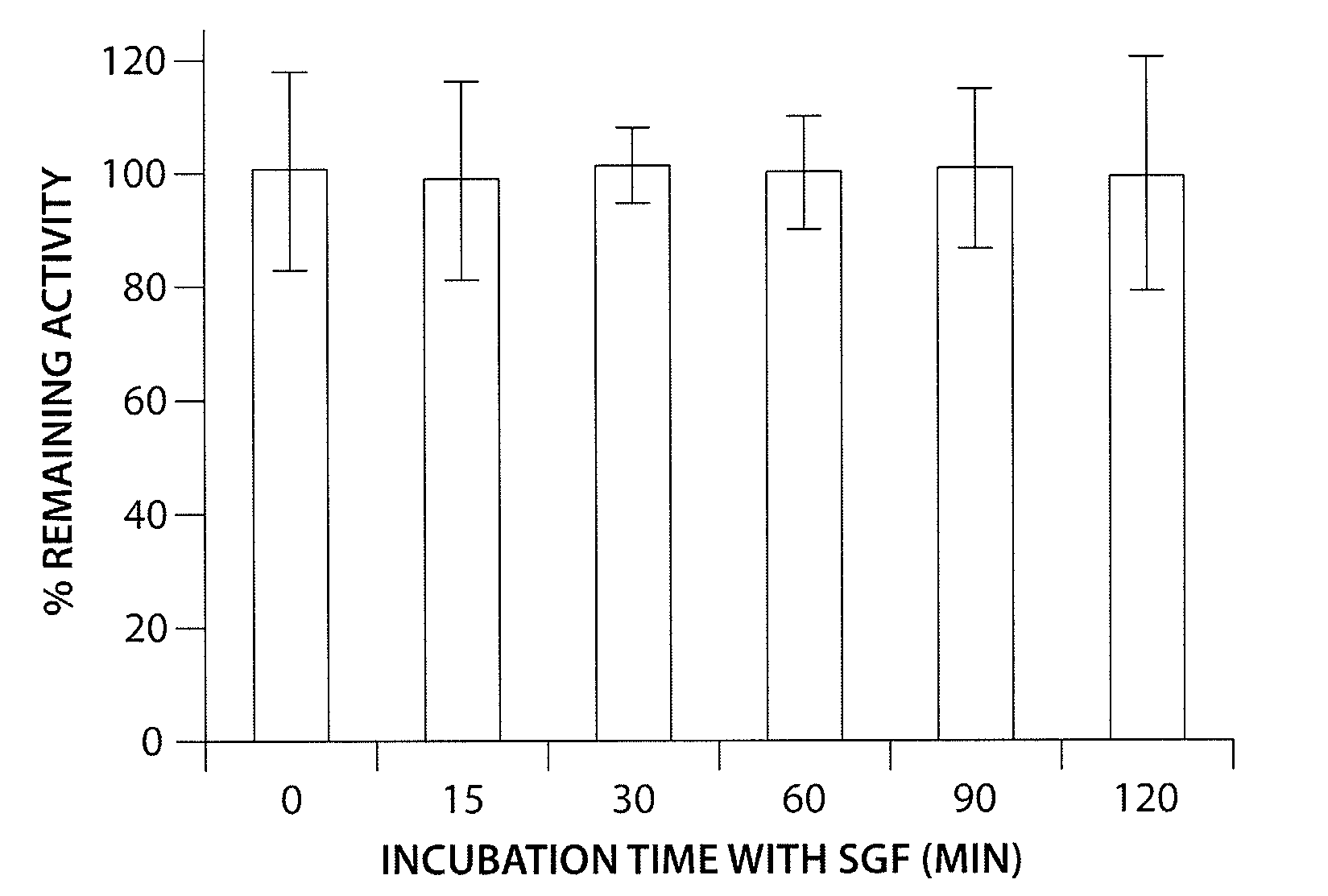
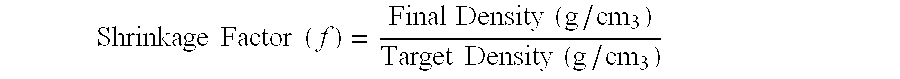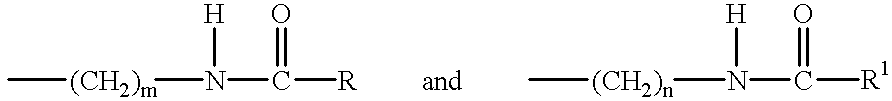Patents
Literature
482results about How to "Excellent property" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aerogel-foam composites
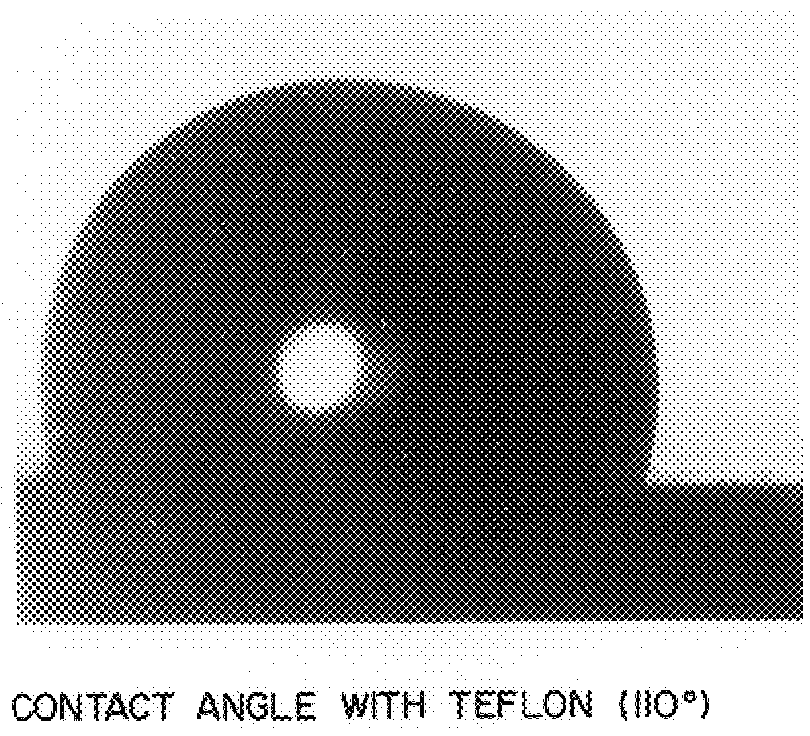
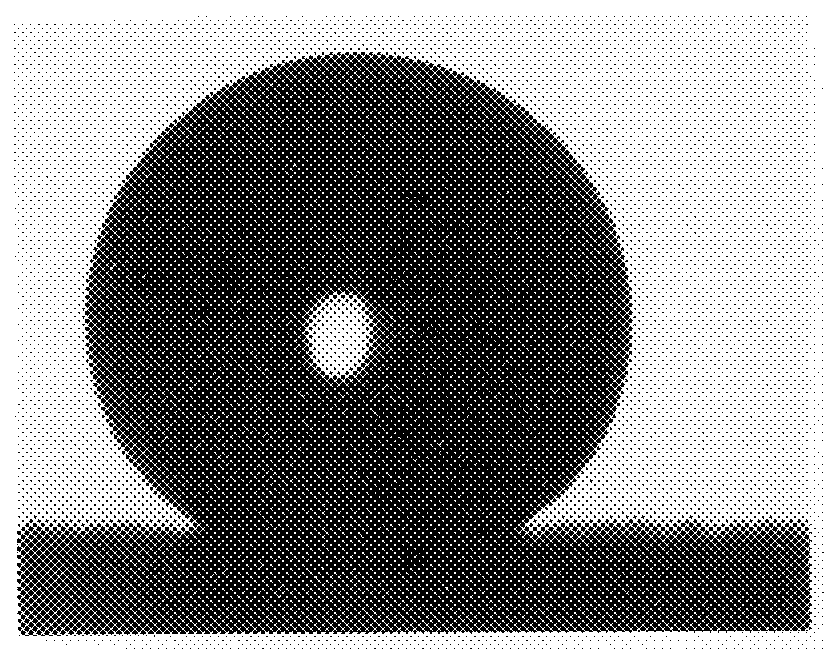
InactiveUS20090029147A1Increase flexibilityMaintain good propertiesSolar heat devicesSynthetic resin layered productsPolymer scienceMonolith
The invention provides reinforced aerogel monoliths as well as reinforced composites thereof for a variety of uses. Compositions and methods of preparing the monoliths and composites are also provided. Application of these materials in transparent assemblies is also discuss.
Owner:ASPEN AEROGELS
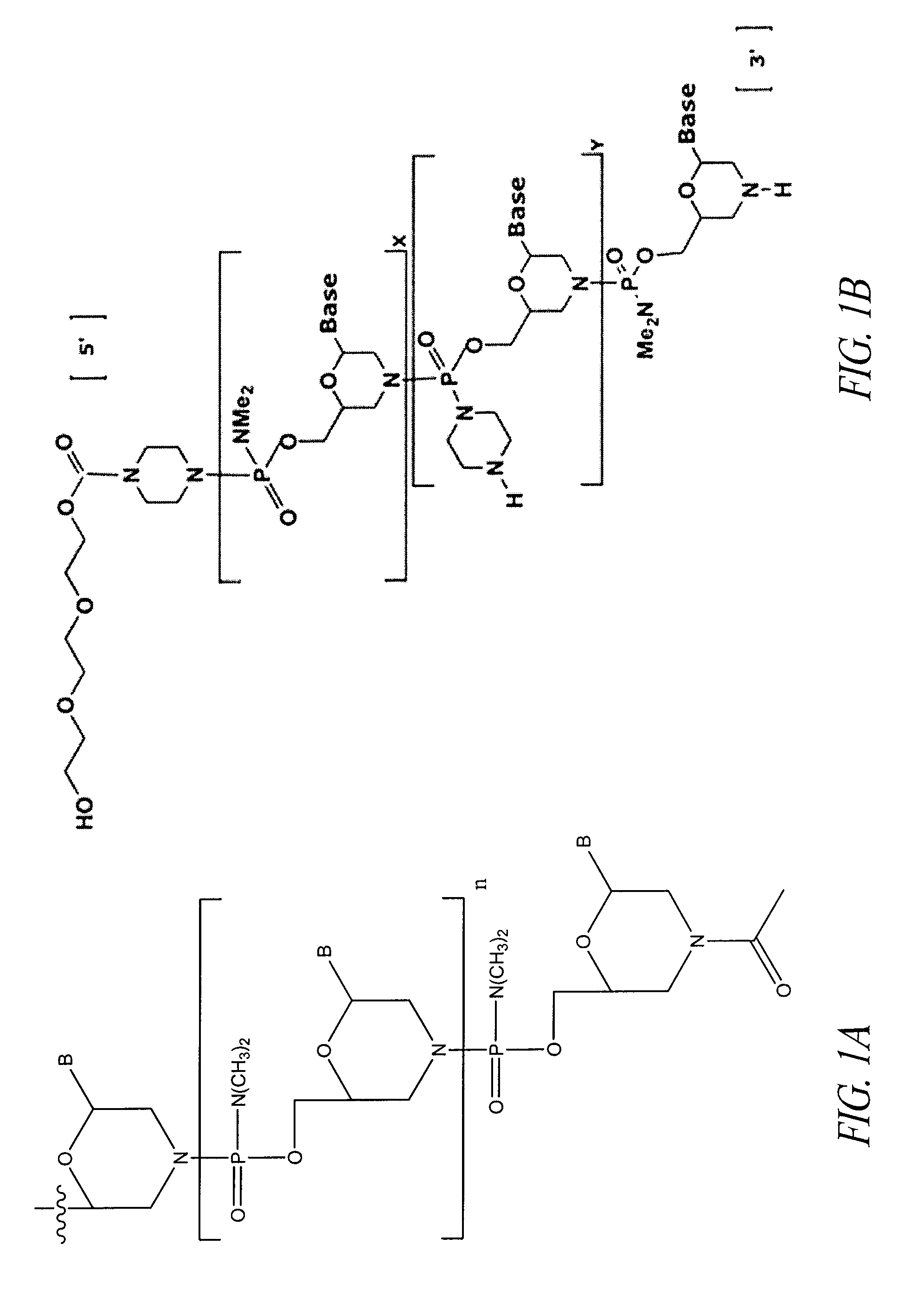
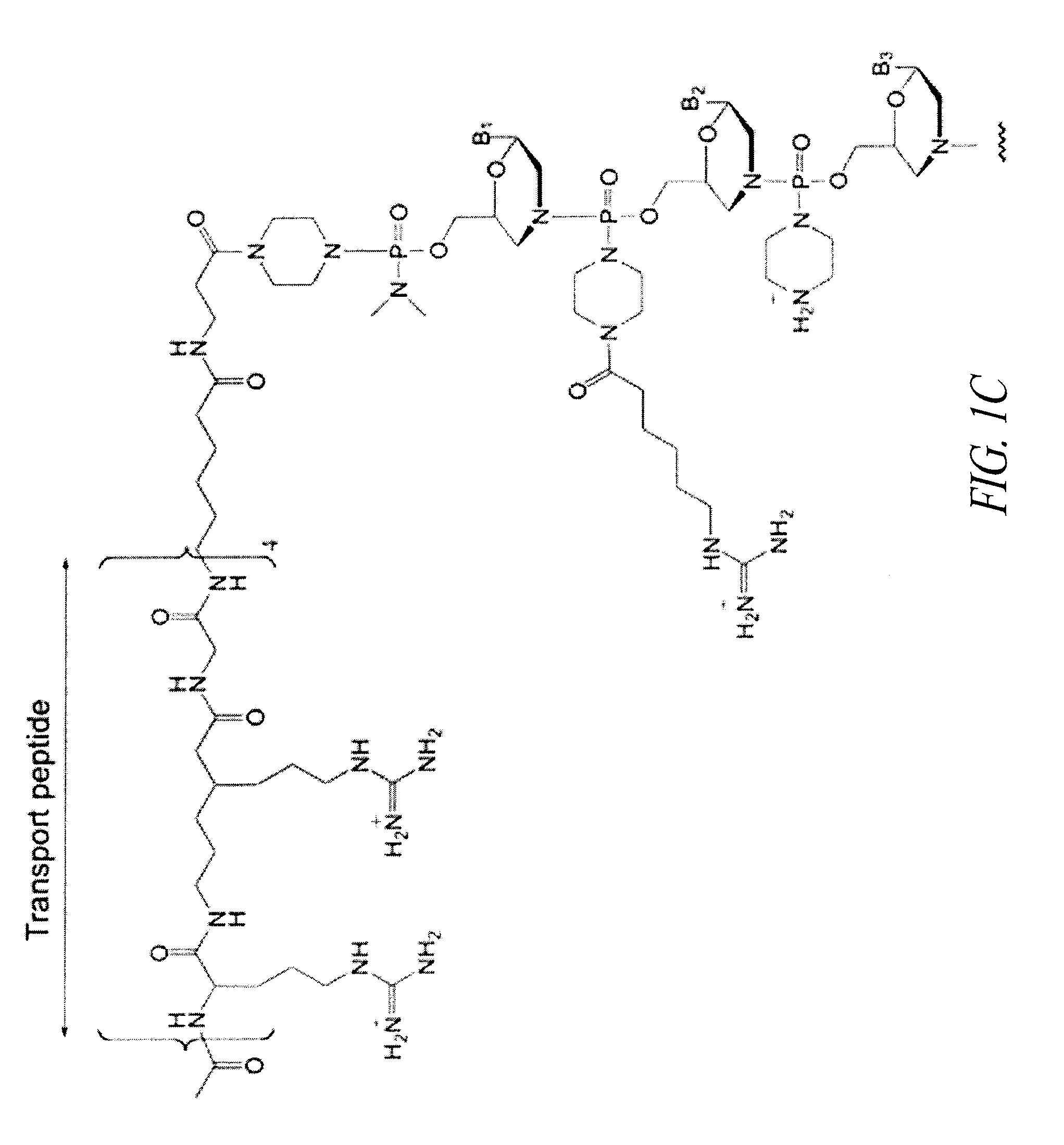
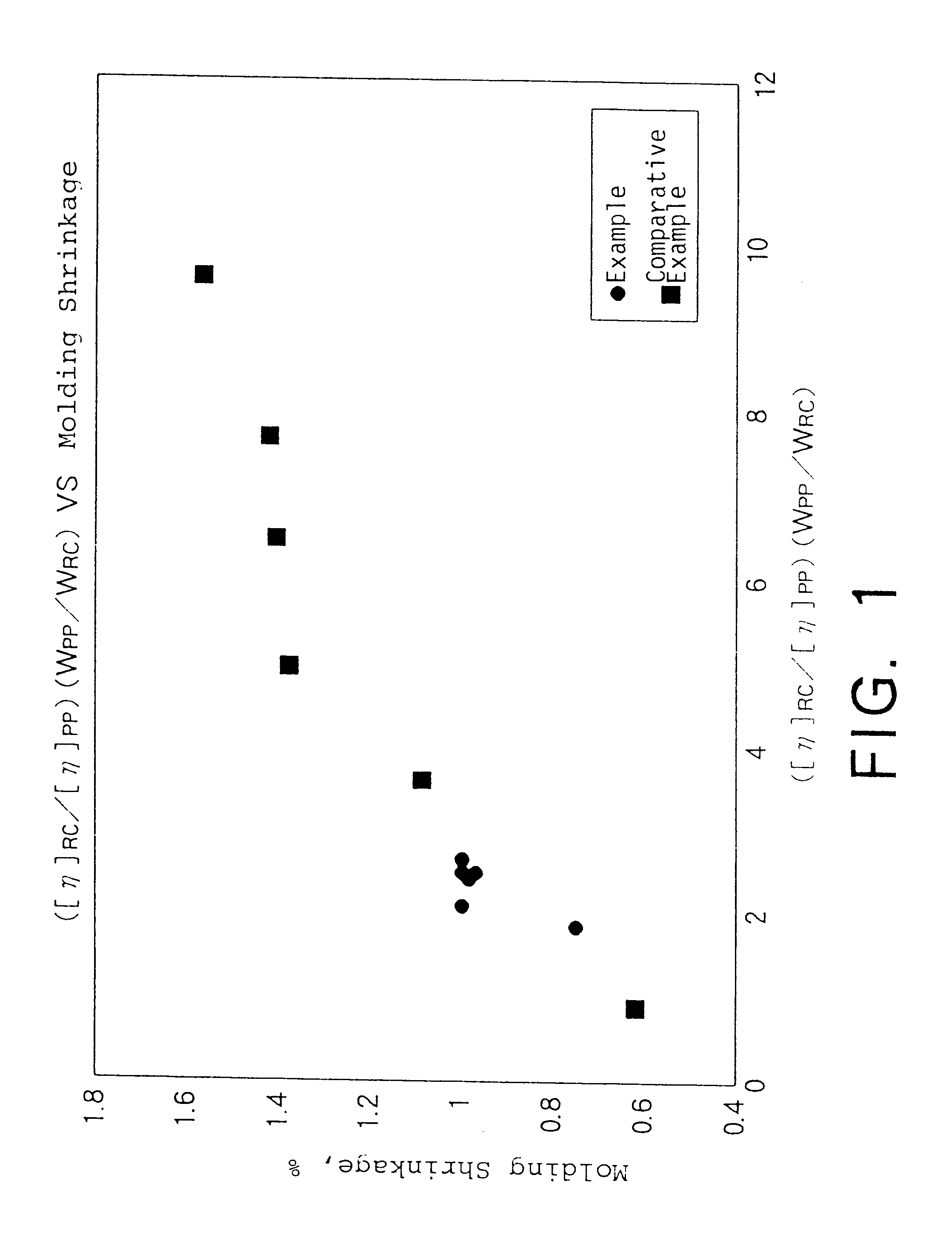
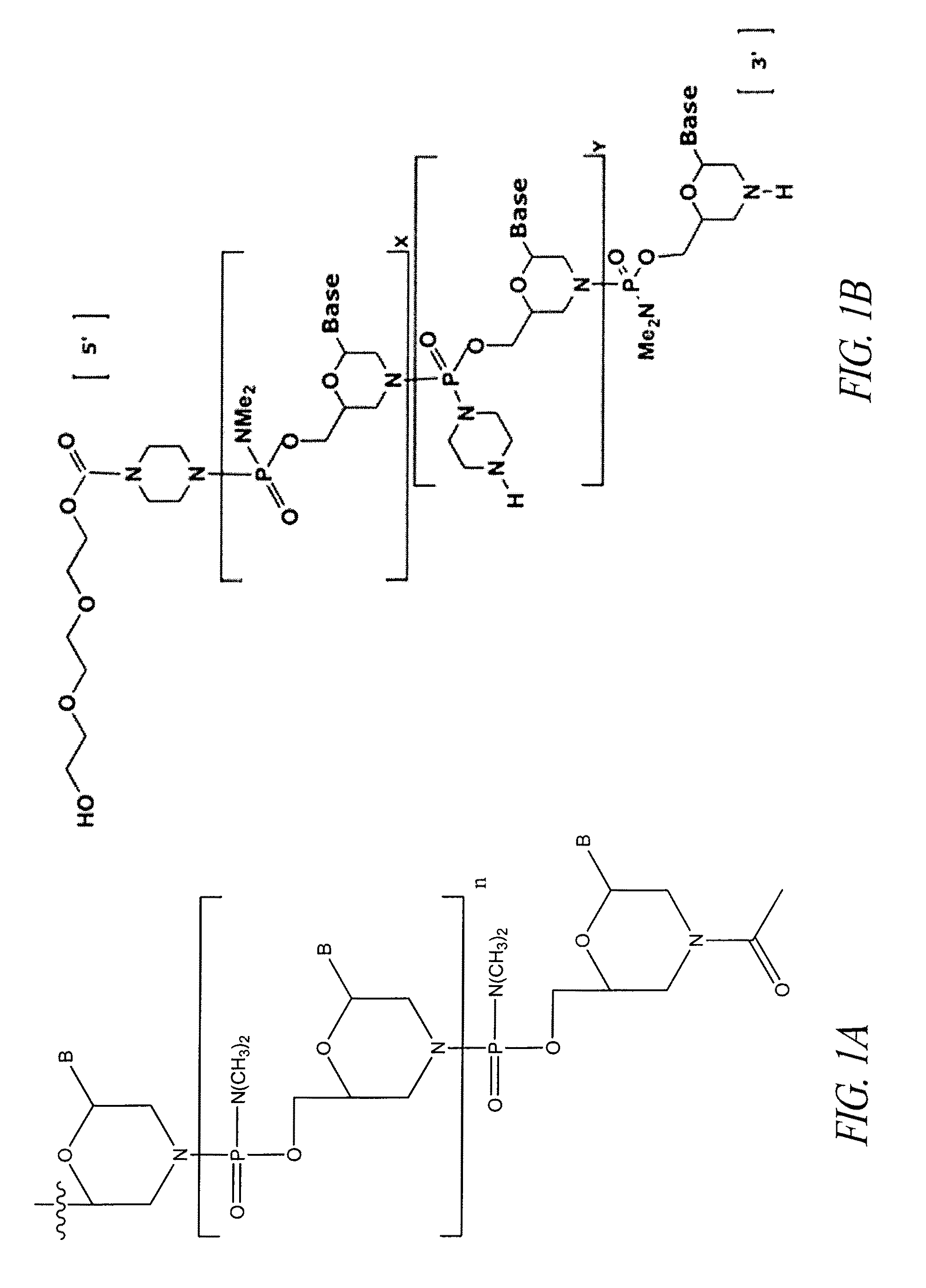
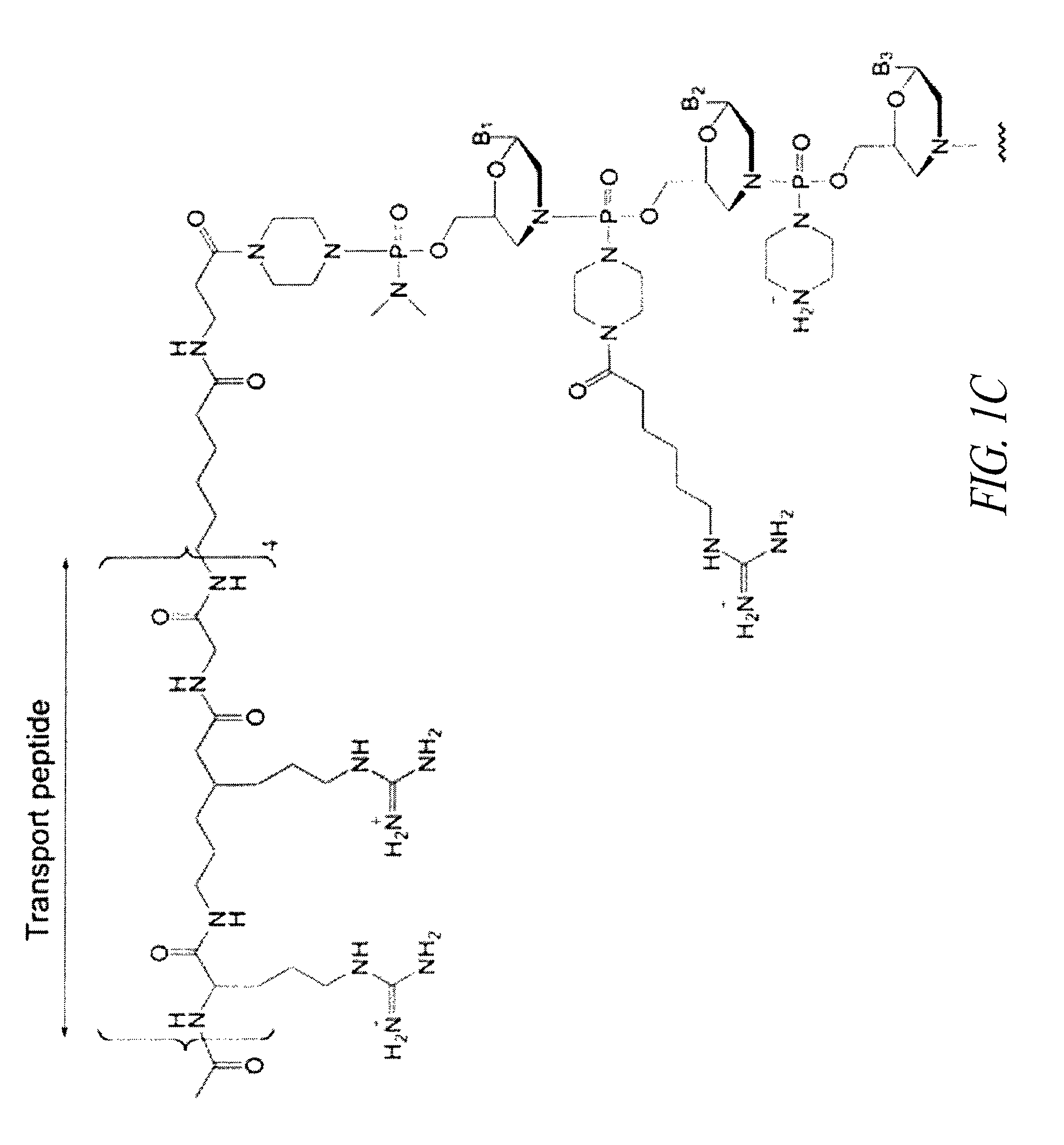
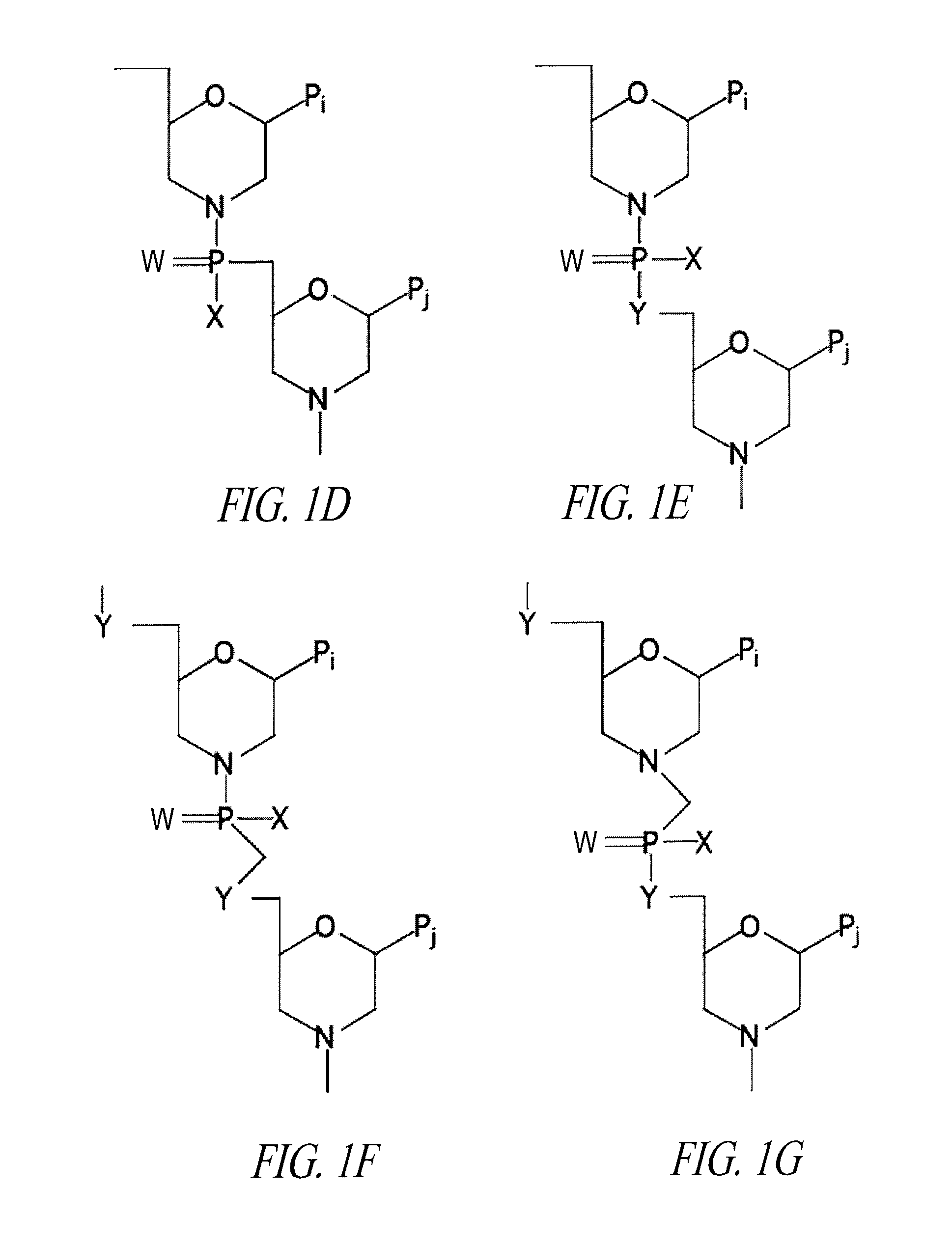
Oligonucleotide analogues having modified intersubunit linkages and/or terminal groups
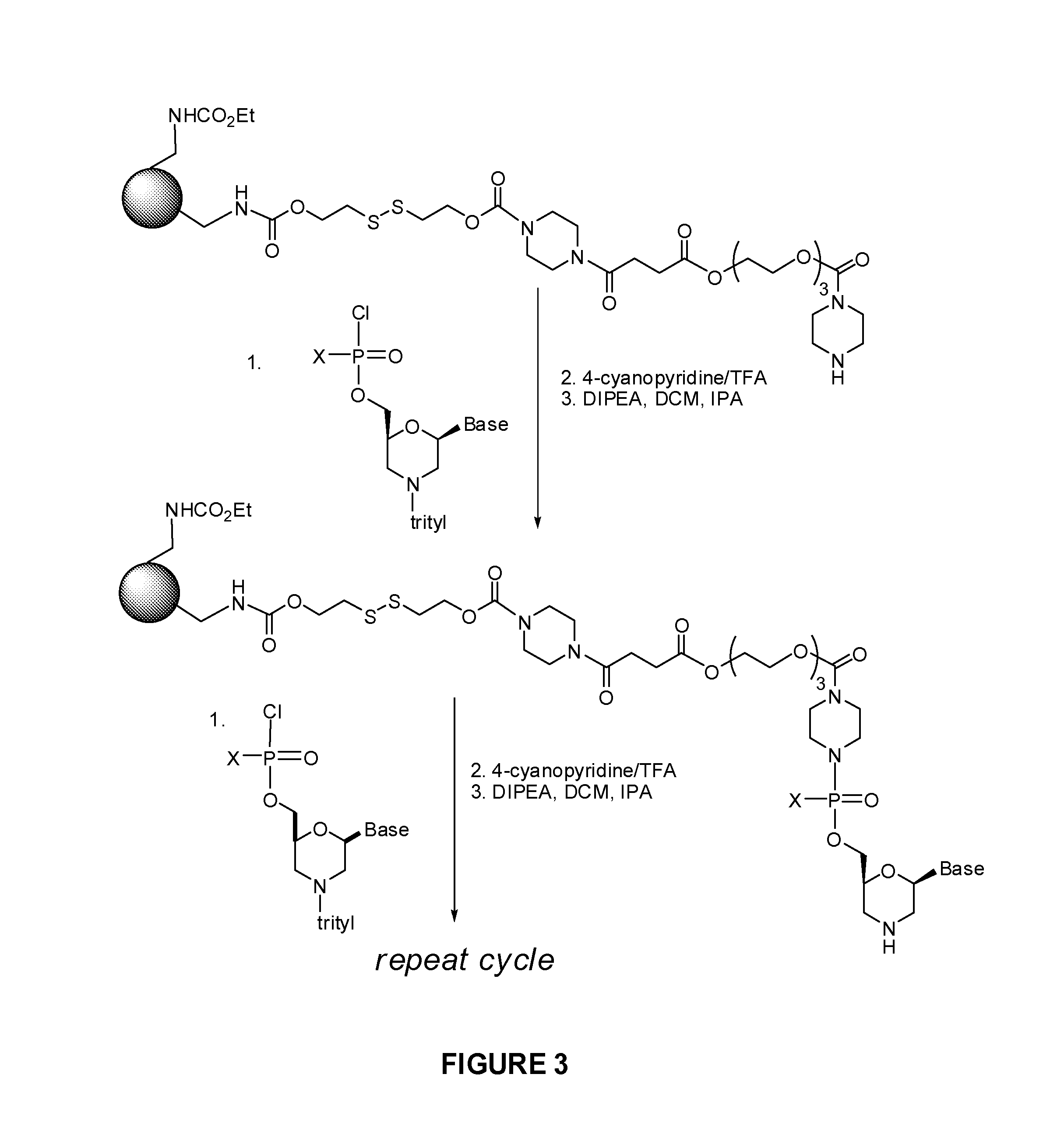
ActiveUS20120065169A1Maintain good propertiesEnhanced cell deliveryAntibacterial agentsBiocideDiseaseEnd-group
Oligonucleotide analogues comprising modified intersubunit linkages and / or modified 3′ and / or 5′-end groups are provided. The disclosed compounds are useful for the treatment of diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
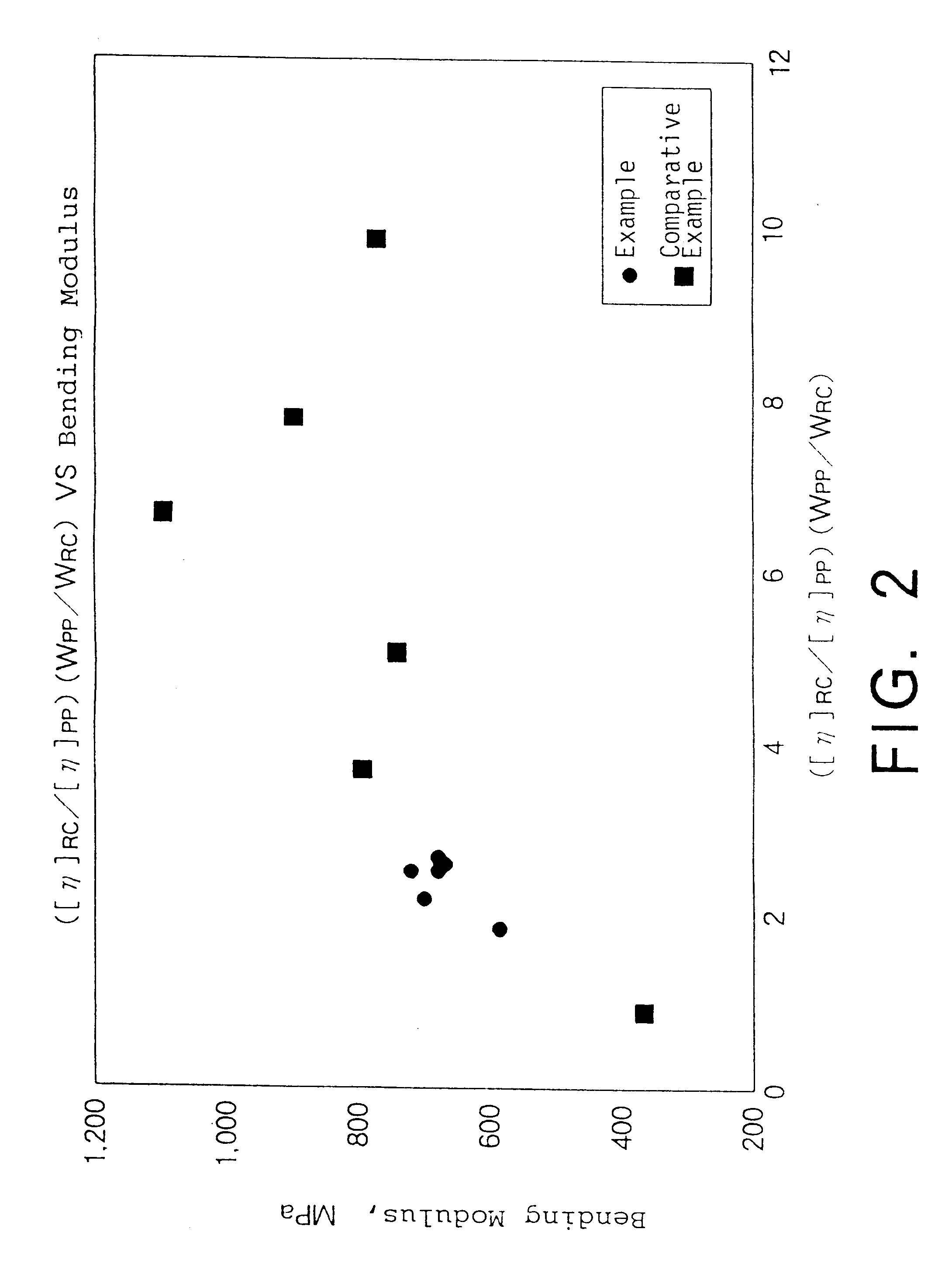
Propylene composition, process for preparing the same, polypropylene composition, and molded articles
InactiveUS6300415B1Excellent propertyImpact resistanceSynthetic resin layered productsWood working apparatusPolypropylenePolyresin
A polypropylene composition for the production of various molded articles which are excellent in moldability, mold shrinkage factor on molding, rigidity, flexibility, impact resistance, in particular low-temperature impact resistance, transparency, gloss, stress-whitening resistance, and the balance thereof; various molded articles having the above properties; a propylene composition which is suitable for a base resin for the polypropylene composition; and a process for the production thereof. The propylene composition comprises a propylene homopolymer and a propylene-ethylene copolymer, the intrinsic viscosity of the copolymer ([eta]RC) is in the range of 1.7 to 2.8 dl / g, the intrinsic viscosity ratio of the homopolymer to the copolymer ([eta]RC / [eta]pp) is in the range of 0.7 to 1.2 and a product [(WPP / WRC)x([eta]RC / [eta]pp)] of the weight ratio (WPP / WRC) of the homopolymer to the copolymer and the intrinsic viscosity ratio thereof is in the range of 1.0 to 3.0.
Owner:JNC CORP
Thermal therapeutic method
InactiveUS20060010902A1Excellent propertyGood dimensional stabilityDomestic cooling apparatusLighting and heating apparatusTherapeutic treatmentBiomedical engineering
This invention relates primarily to a therapeutic method comprising the use of a flexible outer cover that allows quick and easy alternating insertions of separate heat and cold packs, and is attached using an adhesive layer or safety pins to a garment at a location on the garment that is in close contact with an injured area of the body when the garment is worm, to provide alternating cold and hot or hot and cold therapeutic treatments and to a contrasting thermal device comprising a flexible outer cover for use in this method, and an article of manufacture comprising said flexible outer cover and other optional elements to form said contrasting thermal device.
Owner:TRINH ALBERT LONG +2
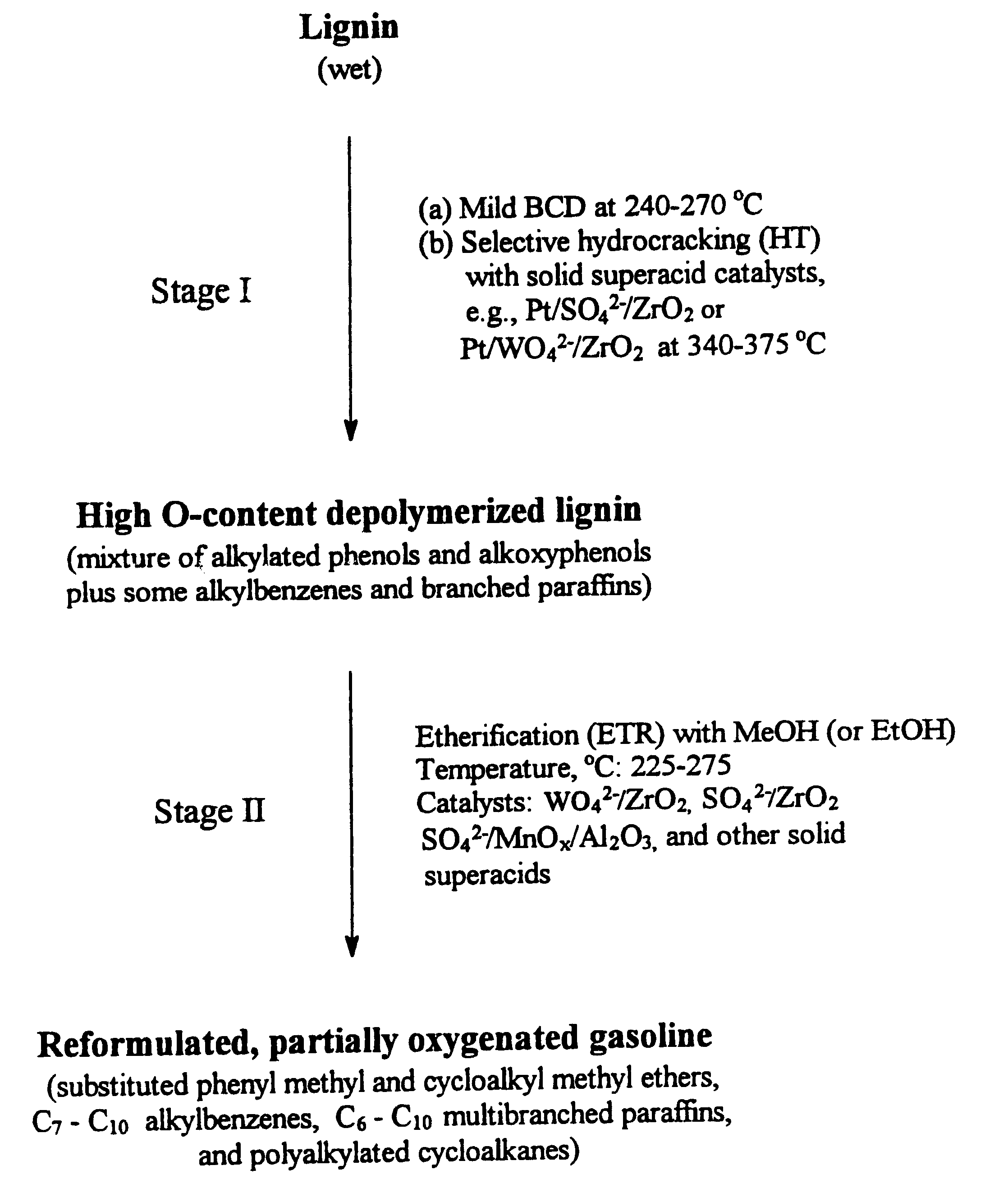
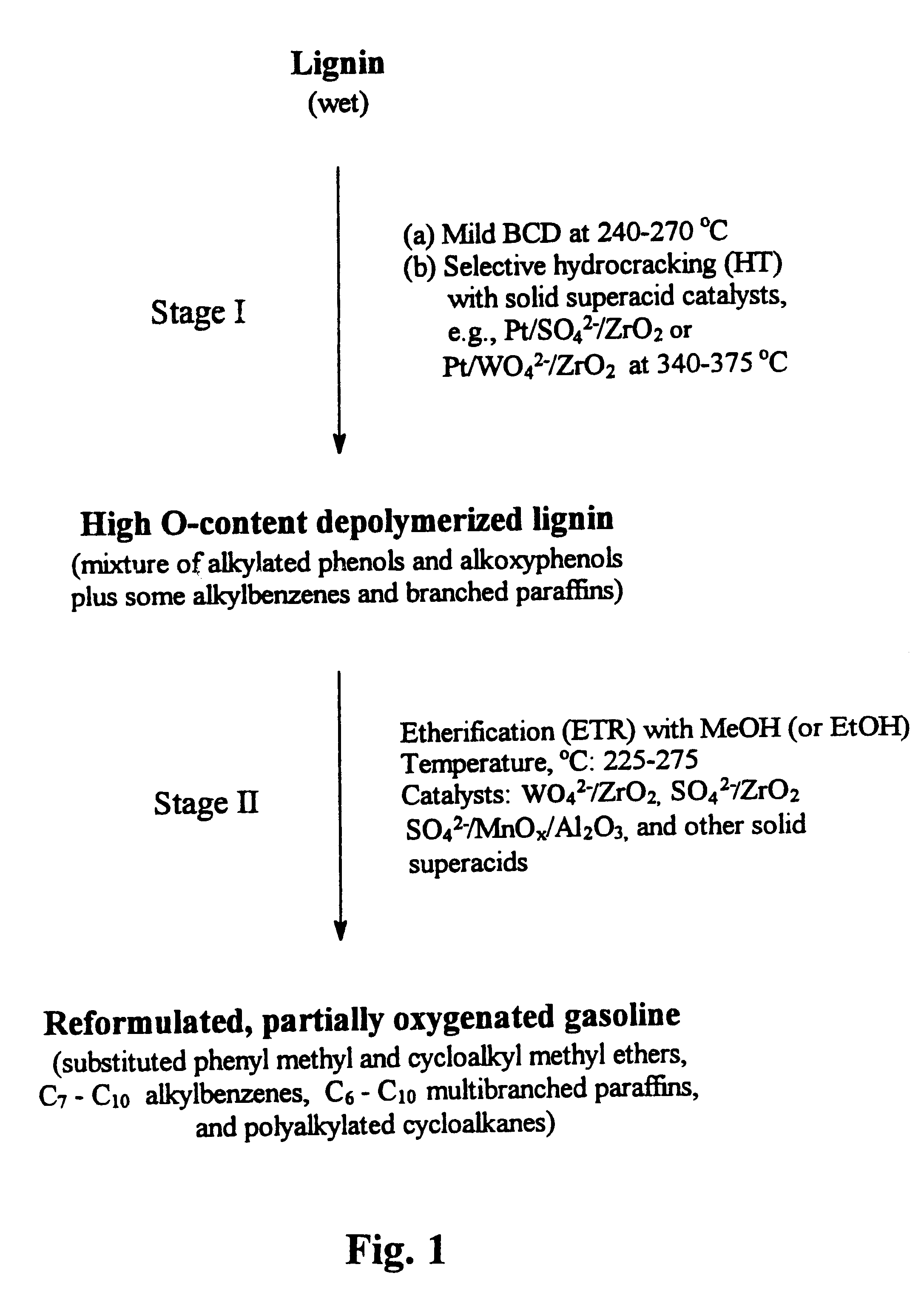
Process for conversion of lignin to reformulated, partially oxygenated gasoline
InactiveUS6172272B1Short reaction timeMaintain good propertiesOrganic compound preparationHydrocarbon from oxygen organic compoundsDepolymerizationMethyl group
A high-yield process for converting lignin into reformulated, partially oxygenated gasoline compositions of high quality is provided. The process is a two-stage catalytic reaction process that produces a reformulated, partially oxygenated gasoline product with a controlled amount of aromatics. In the first stage of the process, a lignin feed material is subjected to a base-catalyzed depolymerization reaction, followed by a selective hydrocracking reaction which utilizes a superacid catalyst to produce a high oxygen-content depolymerized lignin product mainly composed of alkylated phenols, alkylated alkoxyphenols, and alkylbenzenes. In the second stage of the process, the depolymerized lignin product is subjected to an exhaustive etherification reaction, optionally followed by a partial ring hydrogenation reaction, to produce a reformulated, partially oxygenated / etherified gasoline product, which includes a mixture of substituted phenyl / methyl ethers, cycloalkyl methyl ethers, C7-C10 alkylbenzenes, C6-C10 branched and multibranched paraffins, and alkylated and polyalkylated cycloalkanes.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY +1
Polymer endoscopic shaft
ActiveUS20050203341A1Easy to installEnhance detailsSurgeryOptical articlesEngineeringMechanical engineering
An endoscopic shaft having multiple endoscopic elements set in a polymer common housing. The endoscopic elements may be drawn with the common housing to manufacture a component, called an EndoFiber, which forms the length of the endoscopic shaft. A second structure, called an Endocap, may be provided that includes optical elements and which is attached to the end of the EndoFiber to form the shaft.
Owner:PARADIGM OPTICS
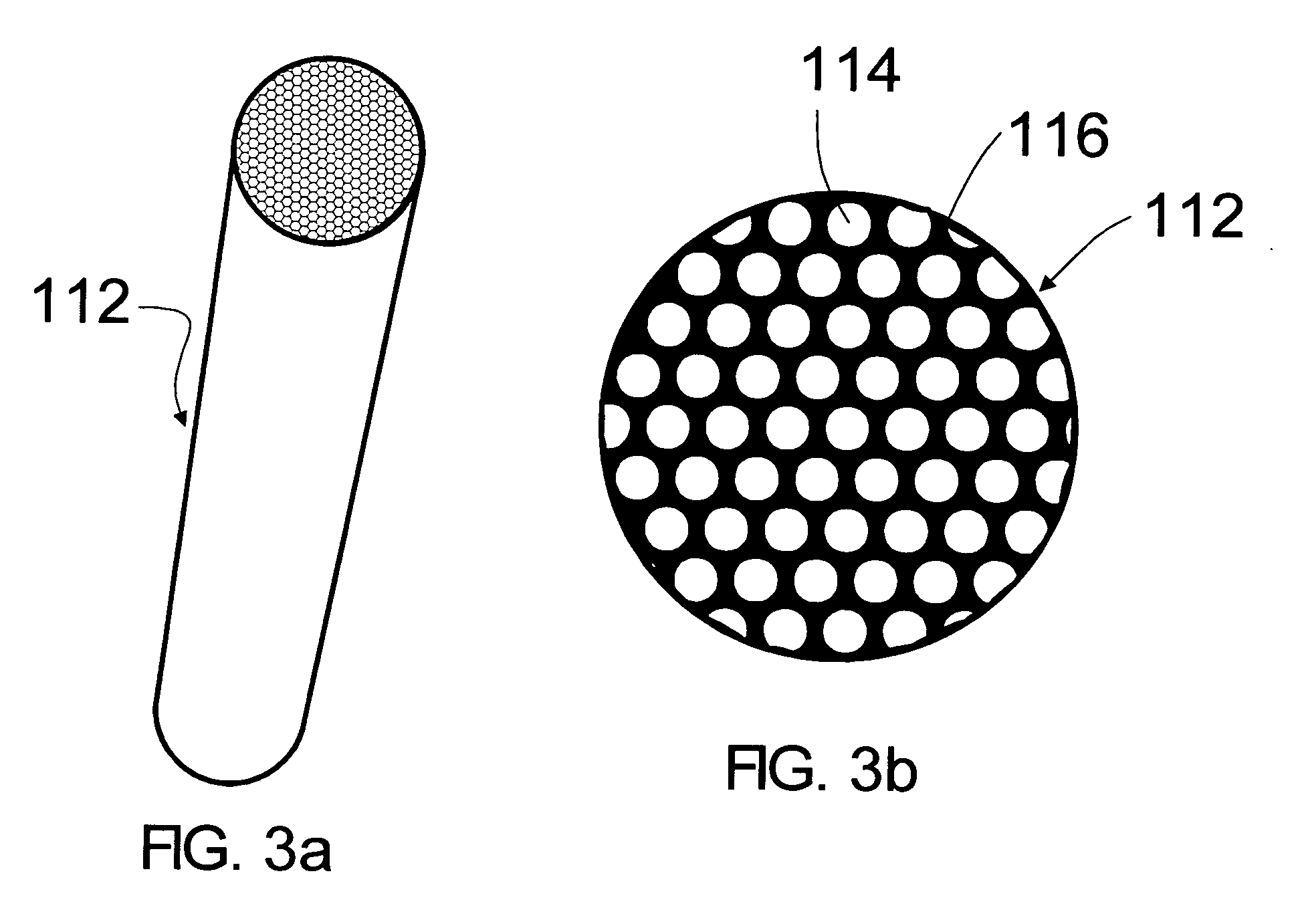
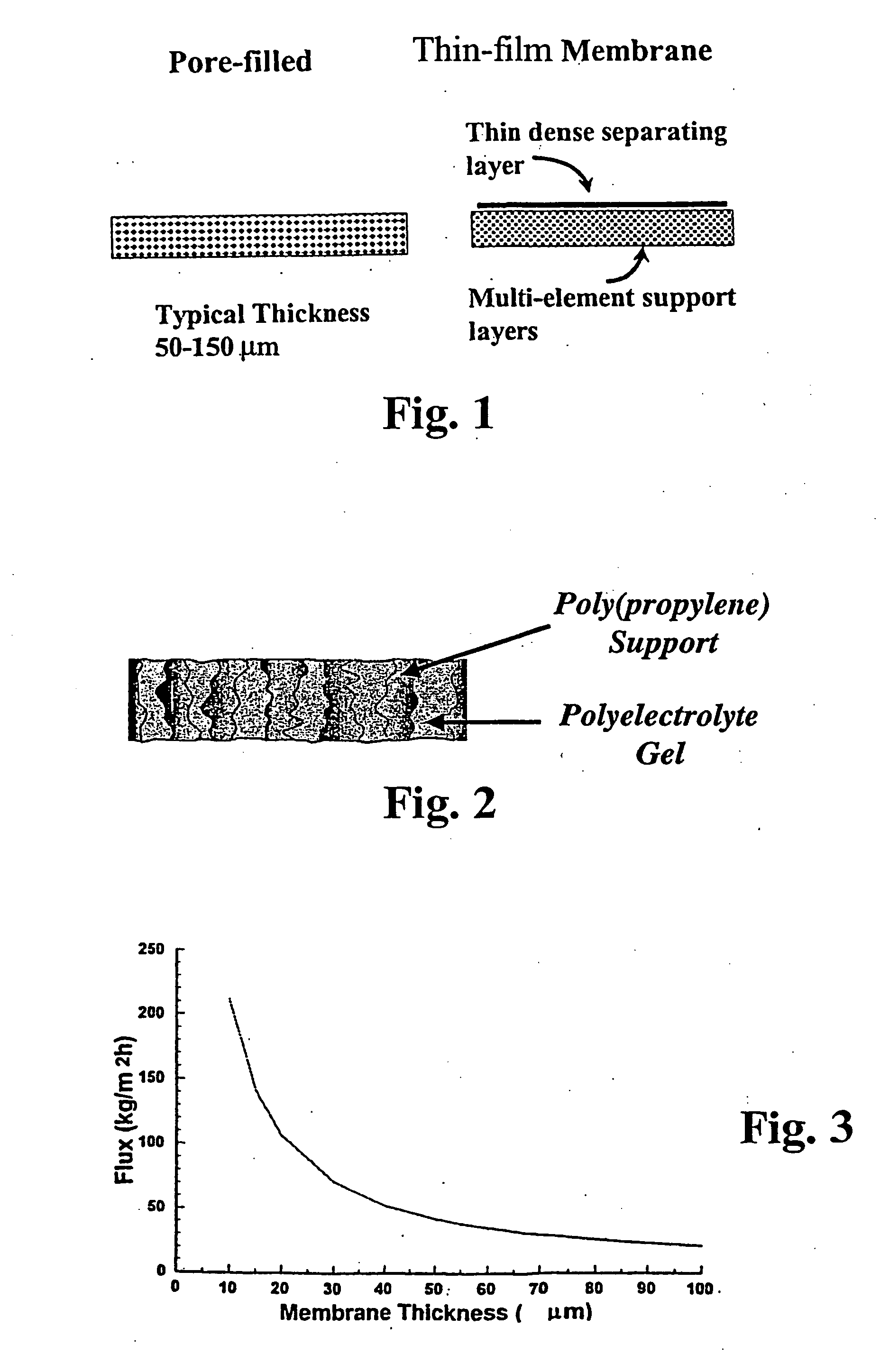
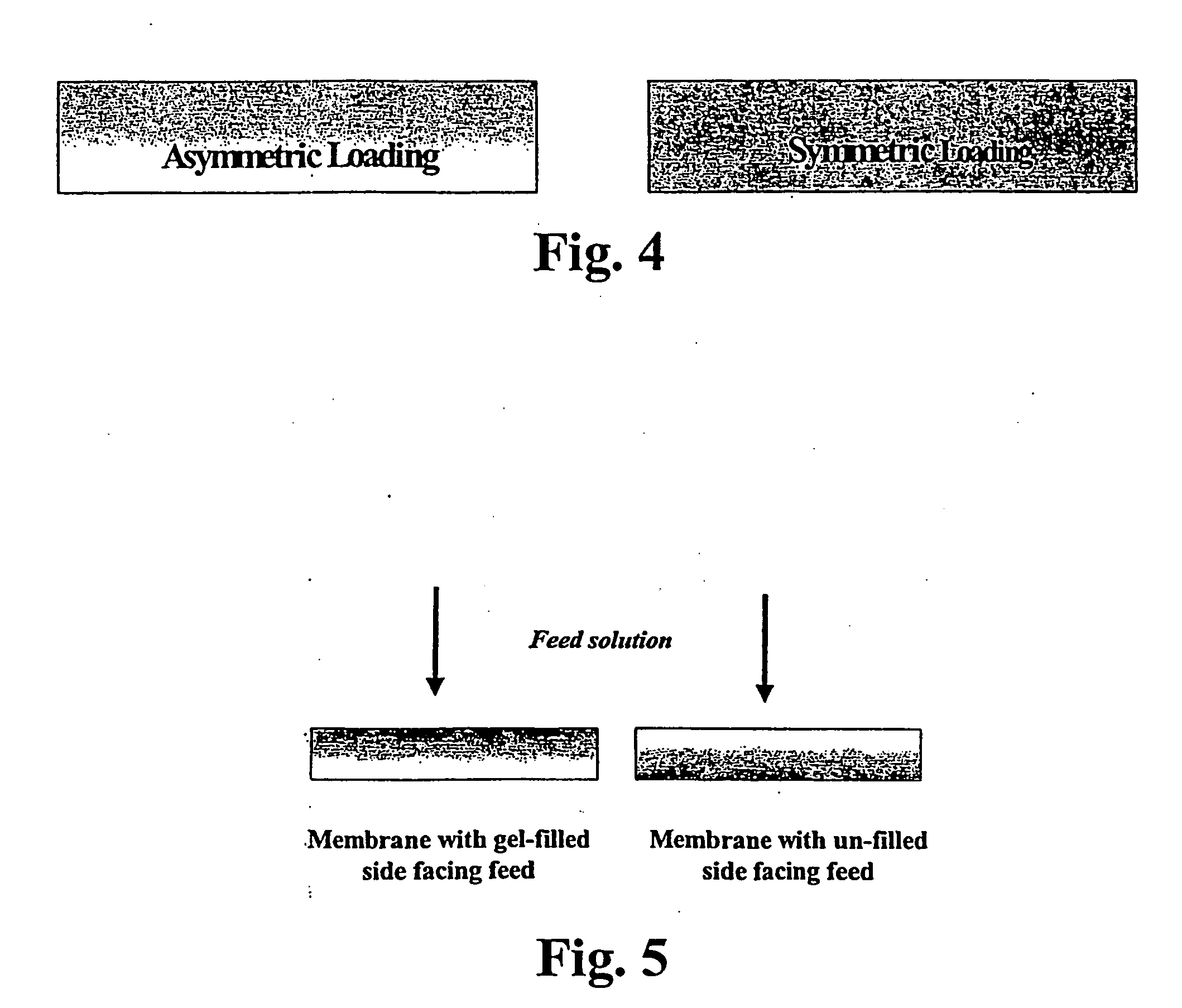
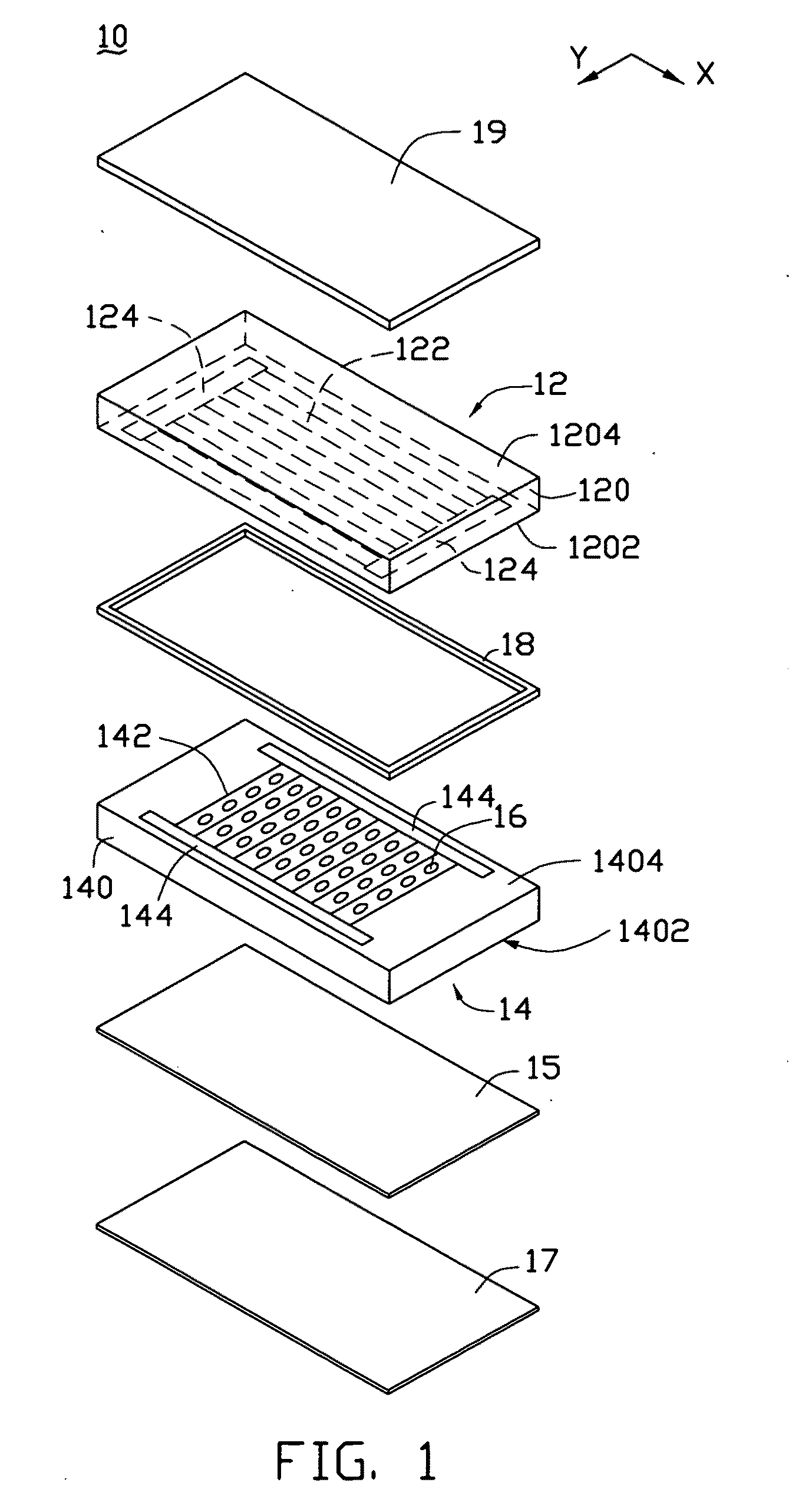
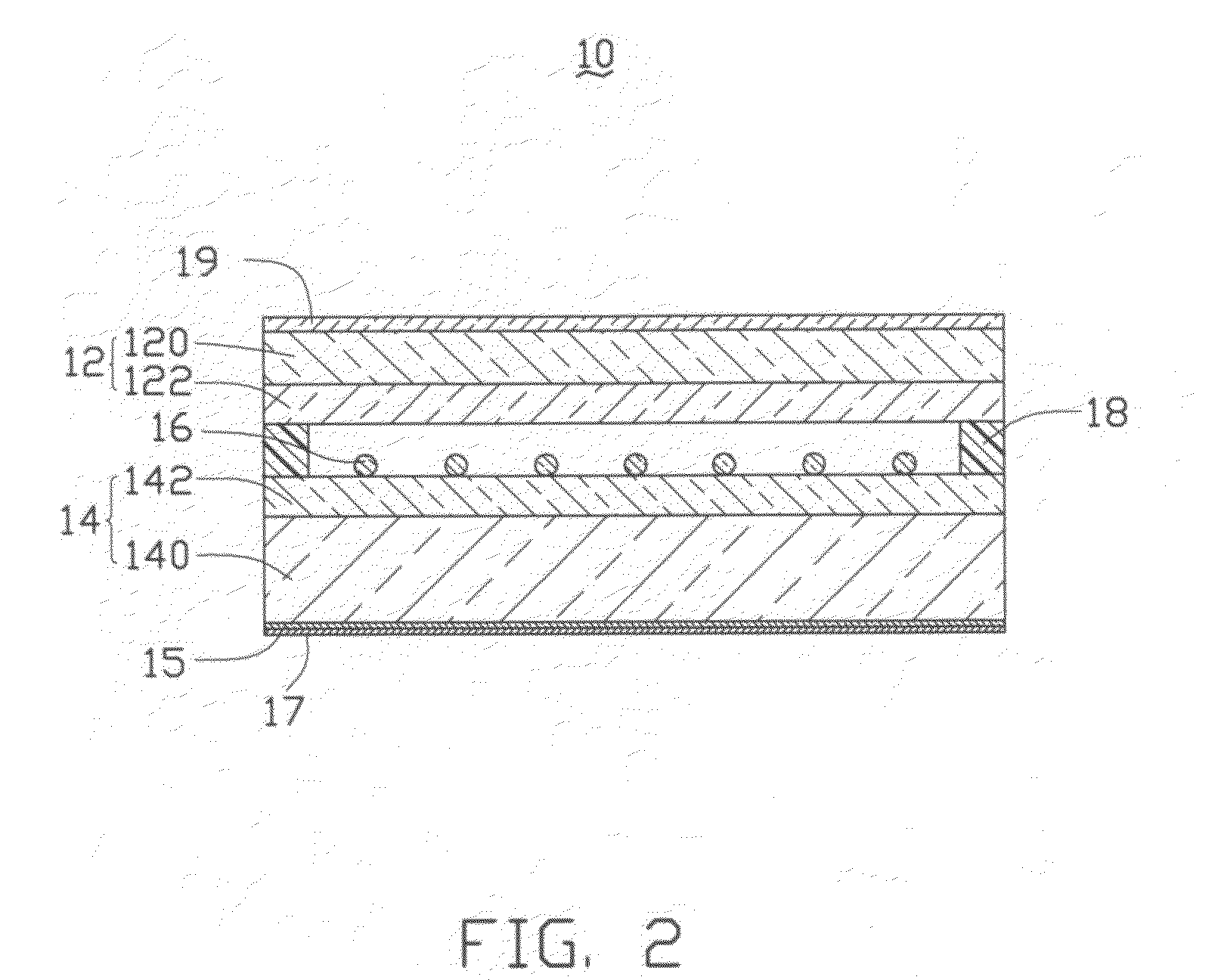
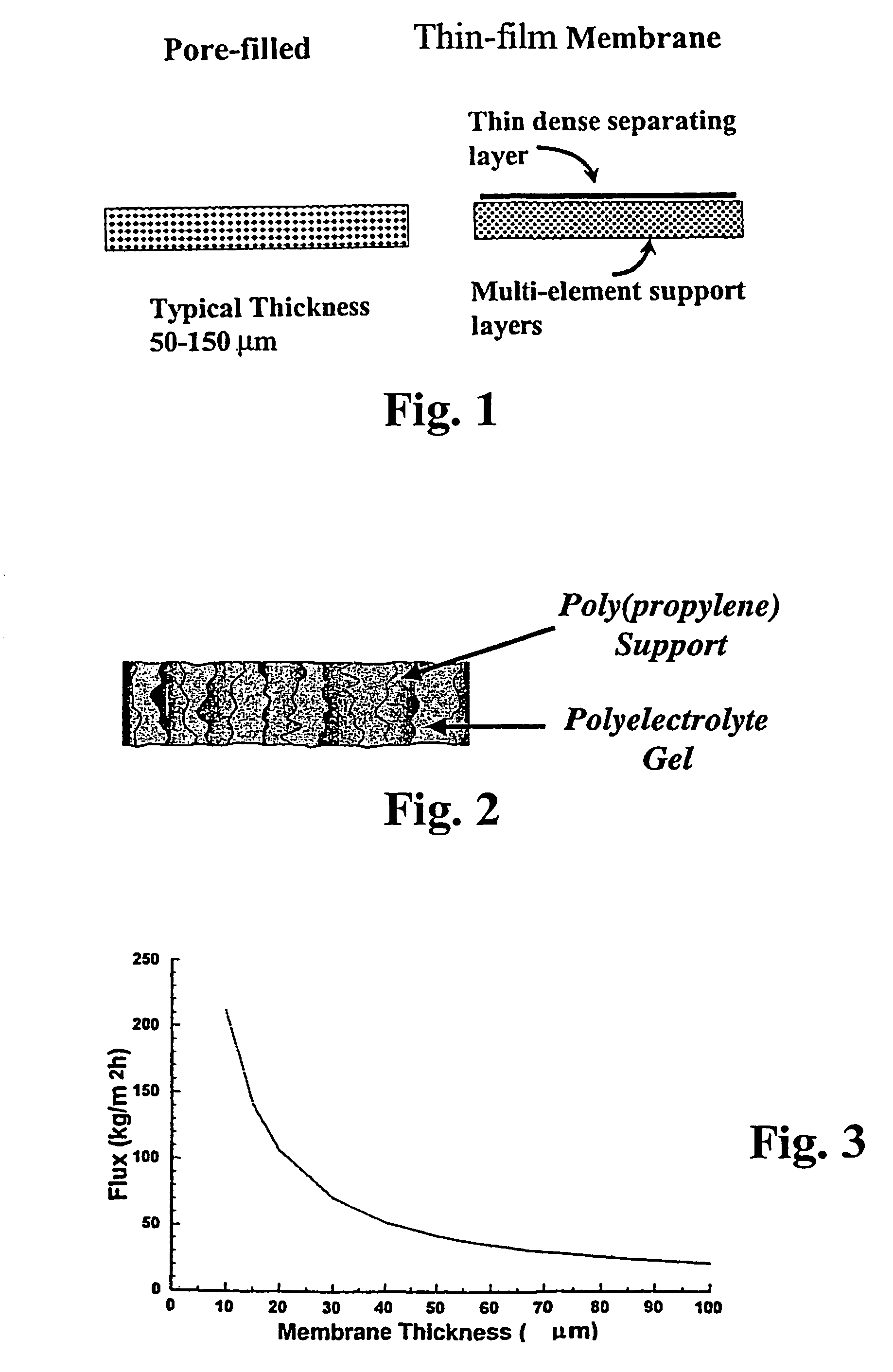
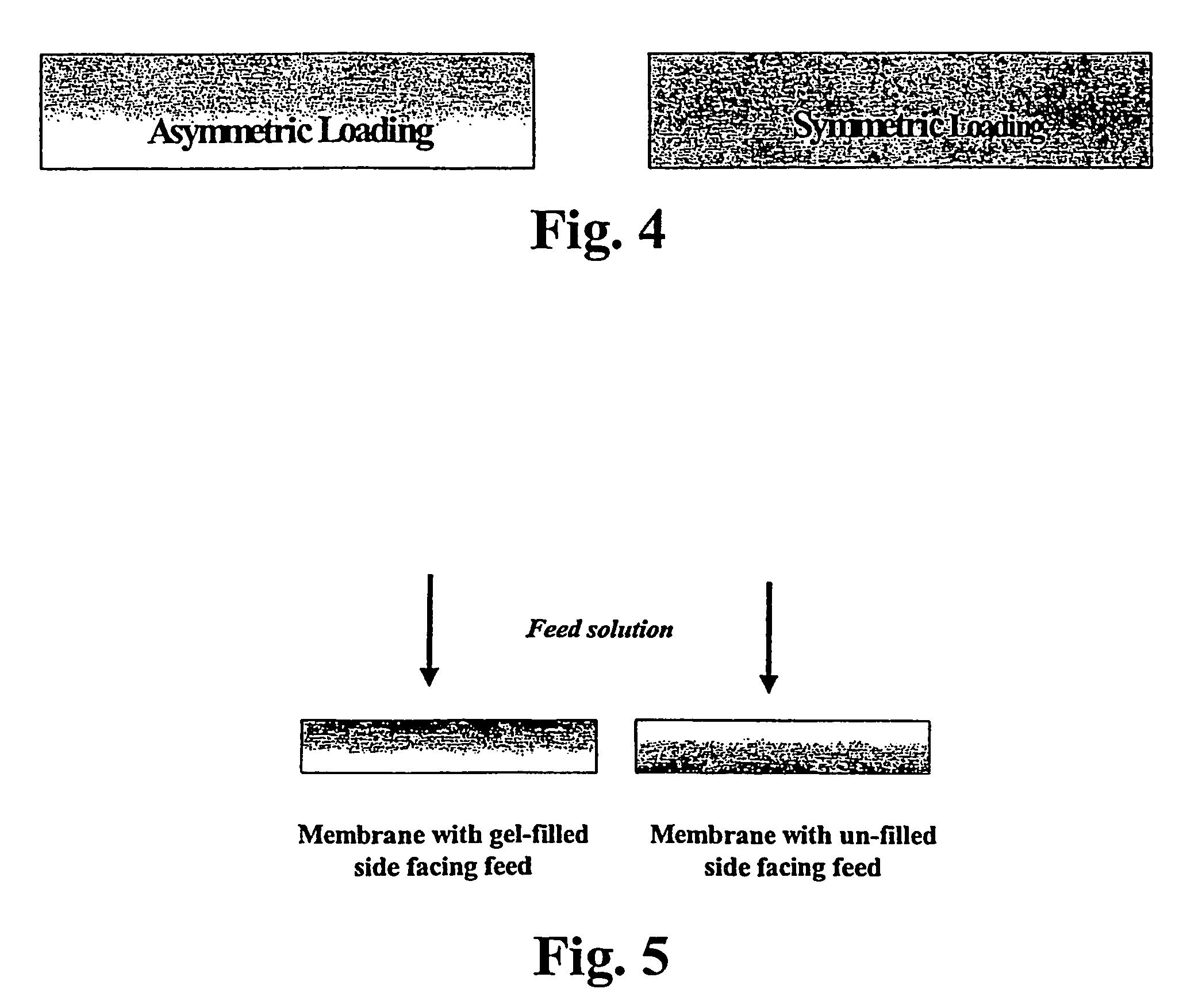
Asymmetric gel-filled microporous membranes
InactiveUS20050011826A1Excellent propertyImprove throughputMembranesSemi-permeable membranesCrystallographyVolumetric Mass Density
The invention provides asymmetric membranes composed of a microporous substrate whose pores contain a crosslinked gel, the density of the crosslinked gel being greater at or adjacent to one major surface of the membrane than the density at the other major surface. The membranes are useful for separating matter from liquids and display good flux and good rejection at low pressure.
Owner:MCMASTER UNIV
Touch panel and display device using the same
ActiveUS20110032196A1Excellent propertyImprove conductivityInput/output processes for data processingDisplay deviceCarbon nanotube
The present disclosure provides a touch panel and a display device employing the same. The touch panel includes at least one transparent layer consisting of a carbon nanotube metal composite layer including a carbon nanotube layer and a metal layer coated on the carbon nanotube layer.
Owner:TSINGHUA UNIV +1
Asymmetric gel-filled microporous membranes
InactiveUS7247370B2Excellent propertyImprove throughputSemi-permeable membranesMembranesCrystallographyVolumetric Mass Density
The invention provides asymmetric membranes composed of a microporous substrate whose pores contain a crosslinked gel, the density of the crosslinked gel being greater at or adjacent to one major surface of the membrane than the density at the other major surface. The membranes are useful for separating matter from liquids and display good flux and good rejection at low pressure.
Owner:MCMASTER UNIV
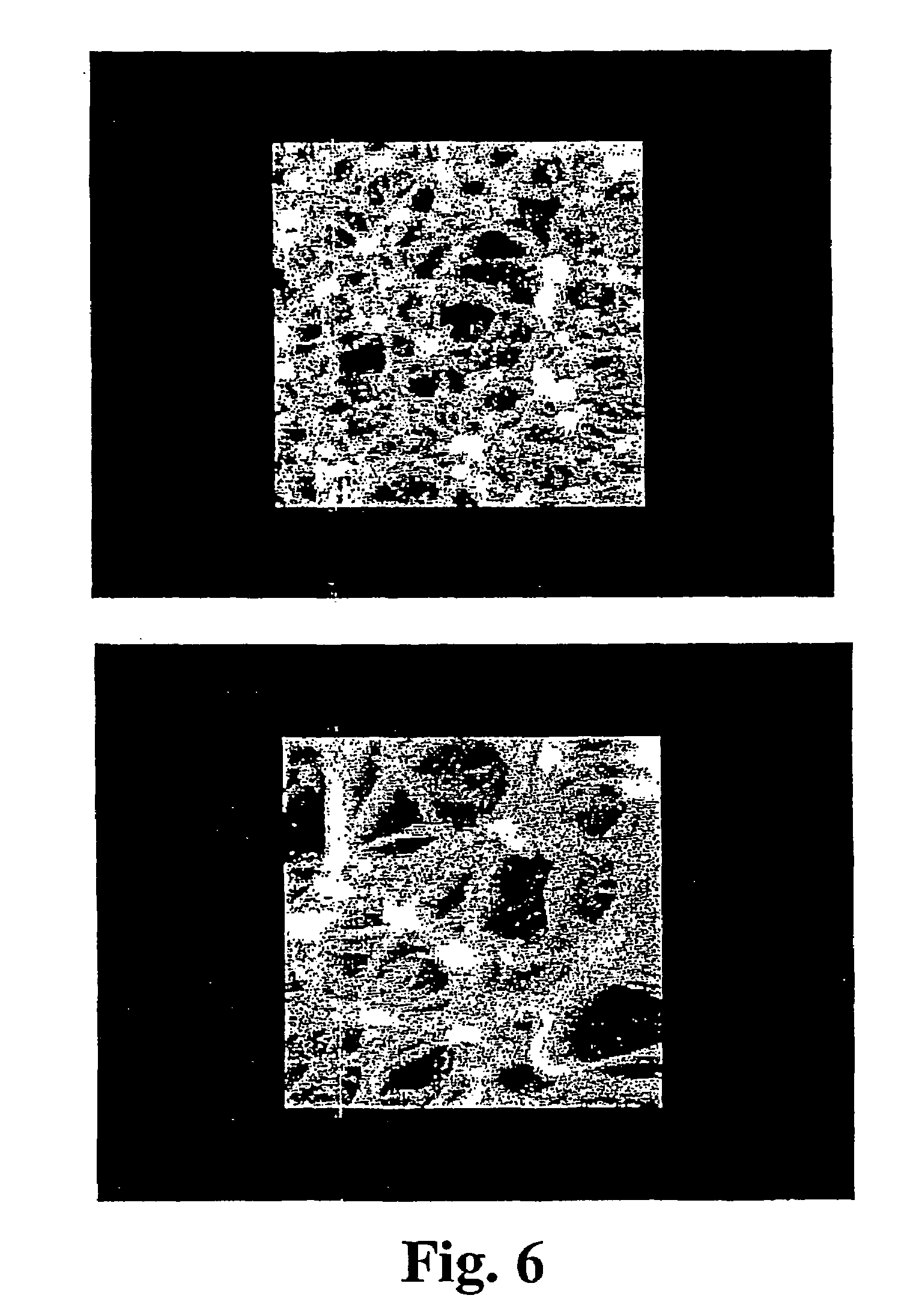
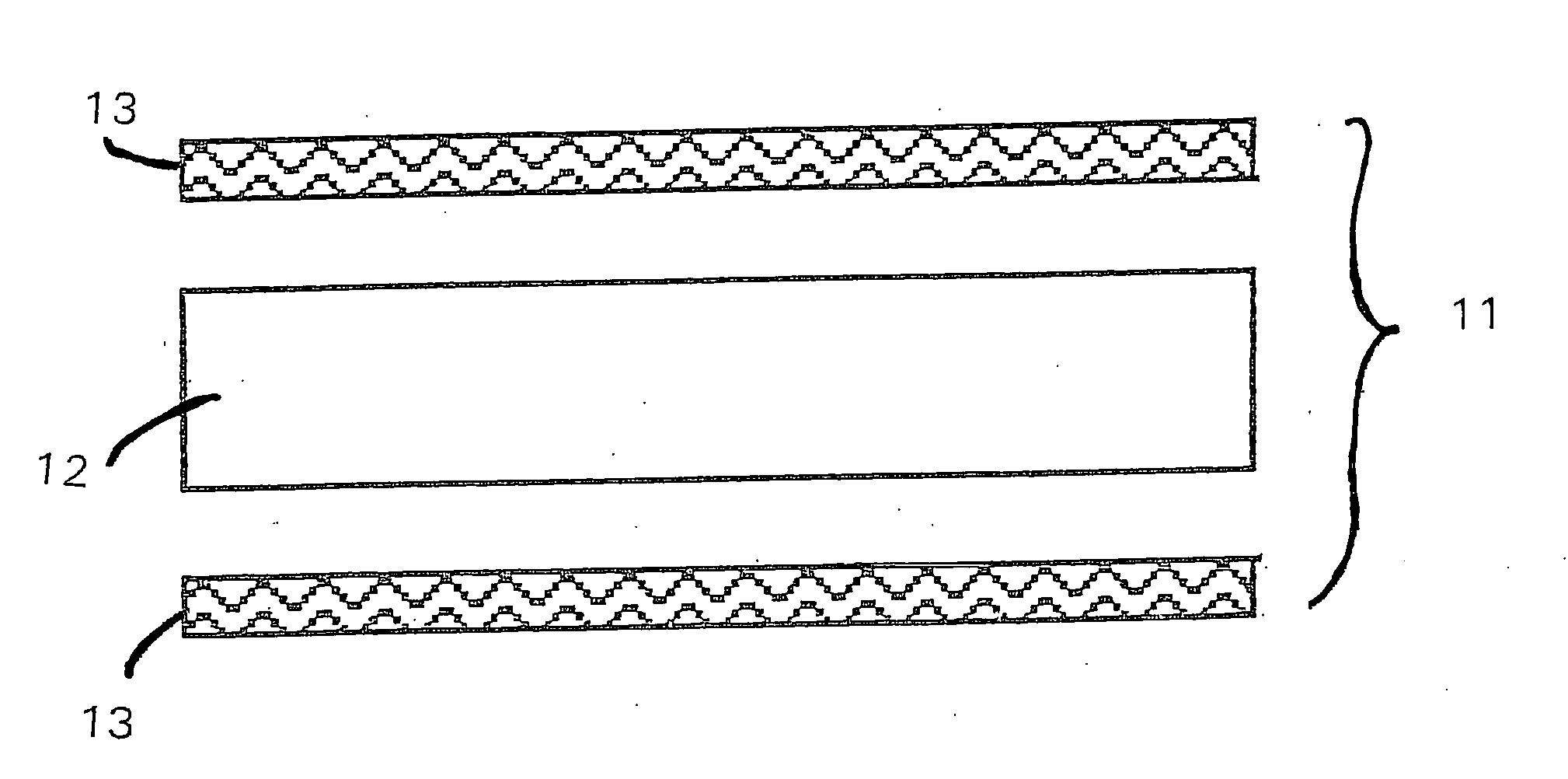
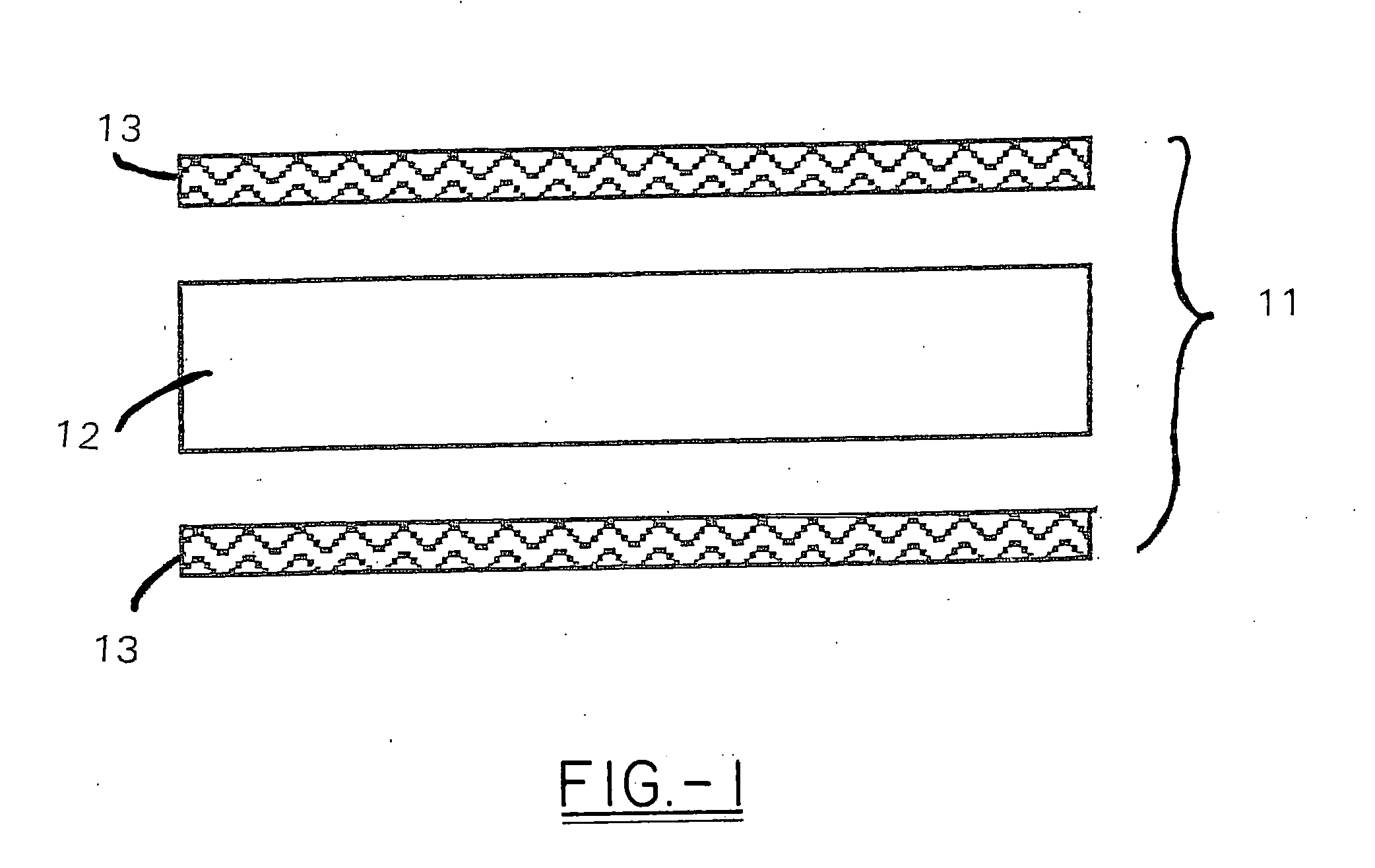
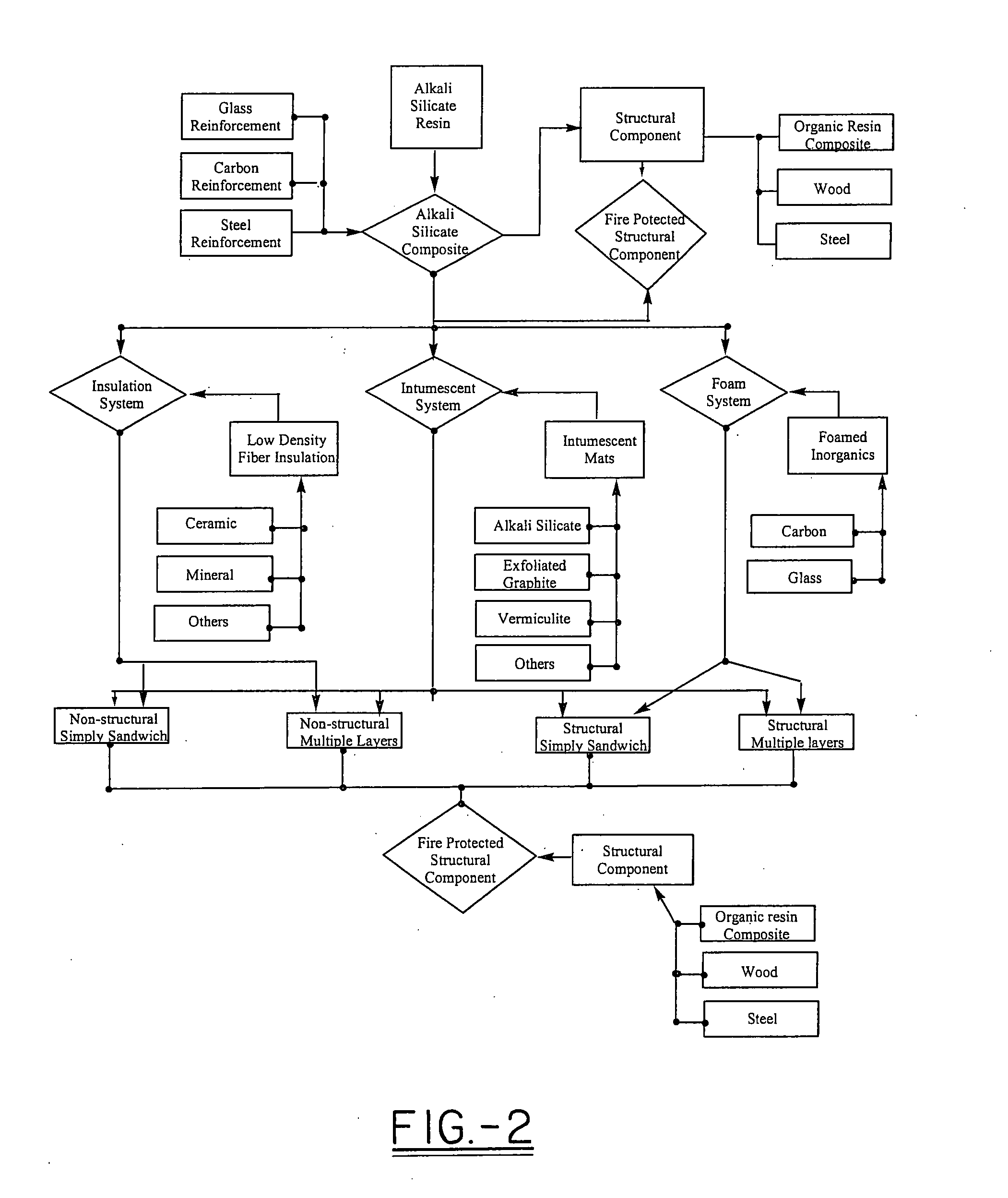
High heat distortion resistant inorganic laminate
InactiveUS20080063875A1High temperature resistanceMaintain good propertiesGlass/slag layered productsNatural mineral layered productsOxyanionPhysical chemistry
A light-weight, heat distortion resistant laminate includes at least one layer of an alkali silicate resin composition derived from an alkali silicate and / or alkali silicate precursor, at least one oxoanionic compound or a reactive acidic glass; and water; and at least one reinforcing core layer comprising an inorganic high-temperature resistant composition. The laminate has a very low quench steepness index value upon rapid quenching as with ambient temperature water.
Owner:THE BF GOODRICH CO
Antiperspirant compositions
InactiveUS6410003B1Excellent propertyLow visible depositCosmetic preparationsToilet preparationsAminalCyclohexane
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
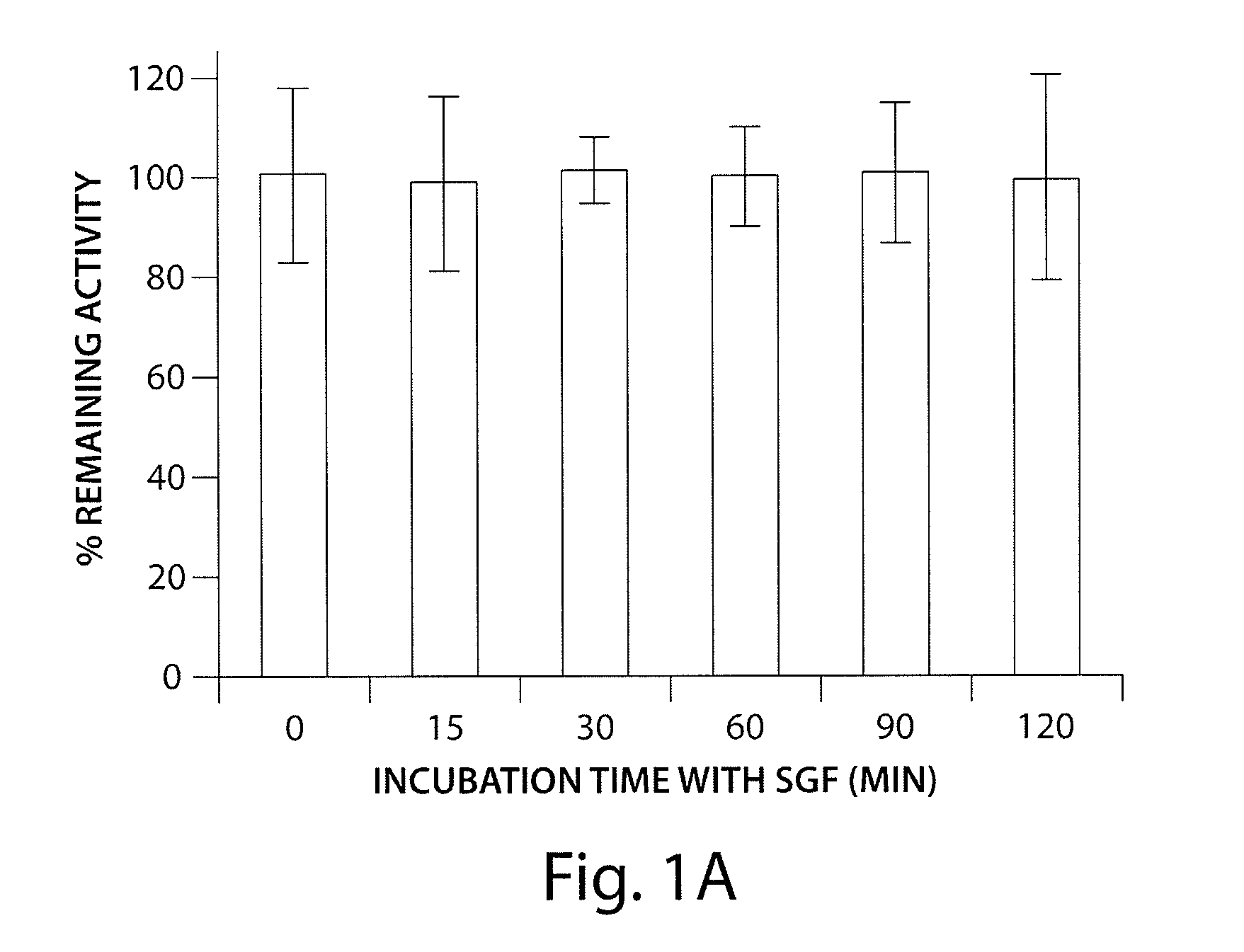
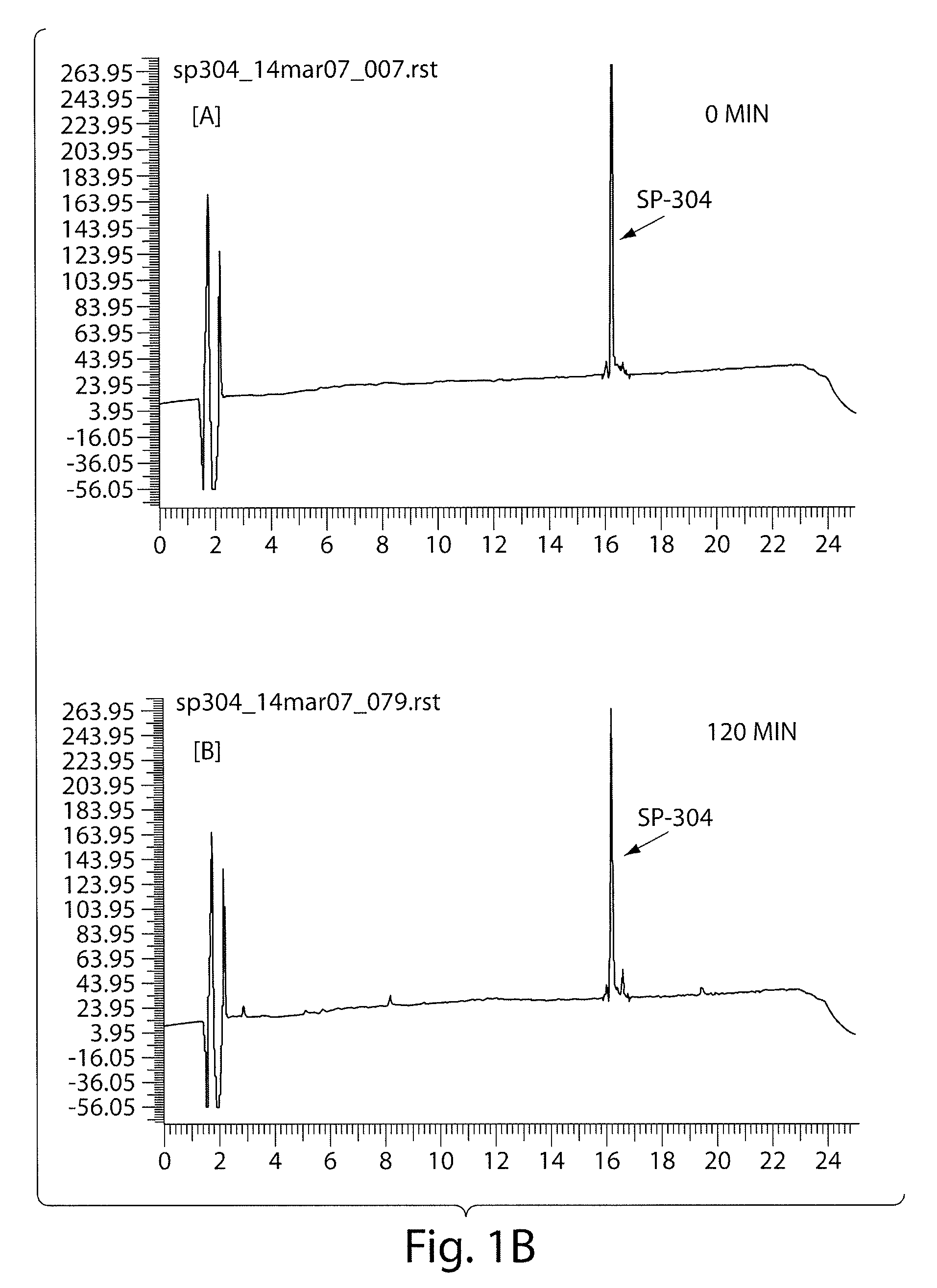
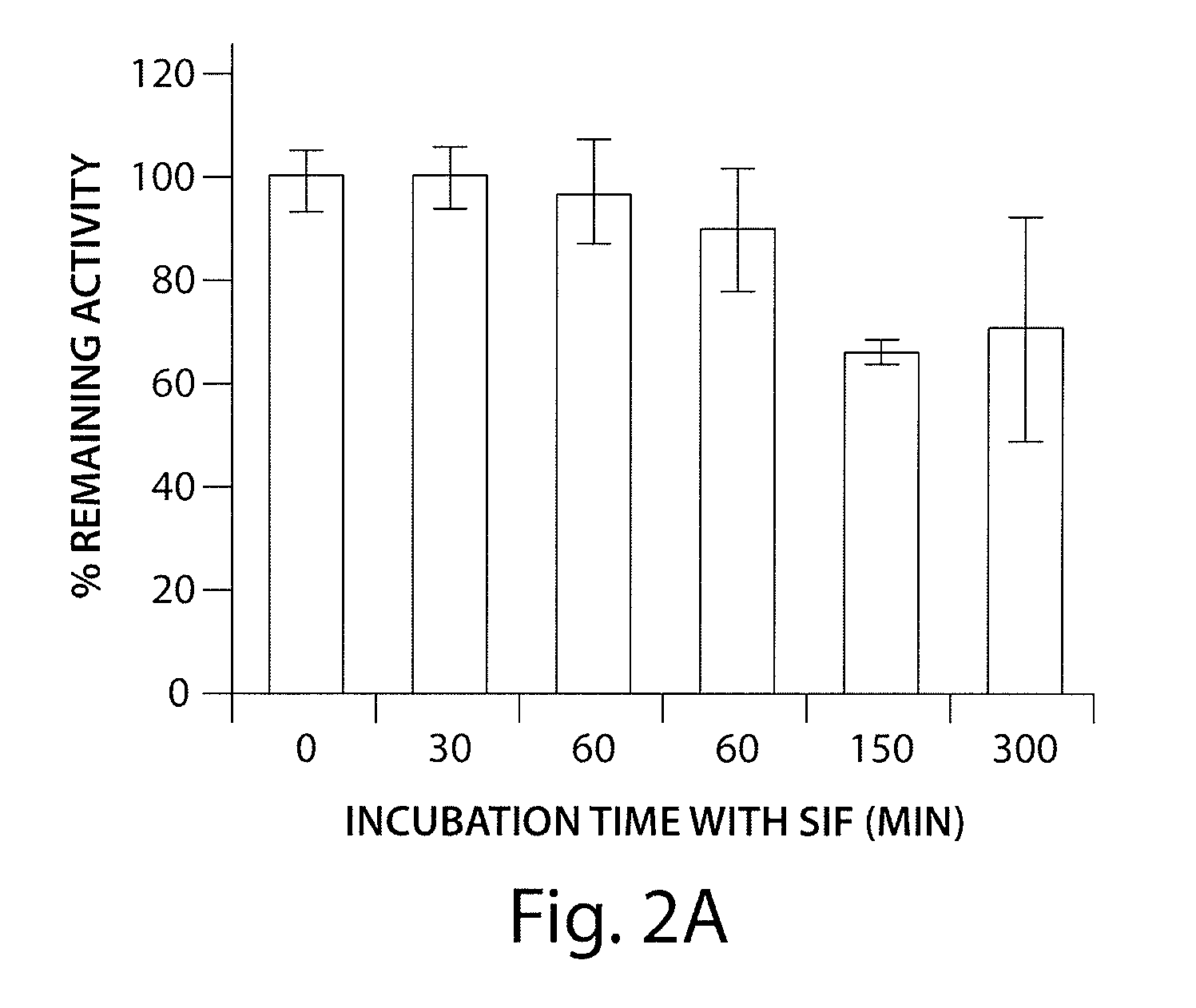
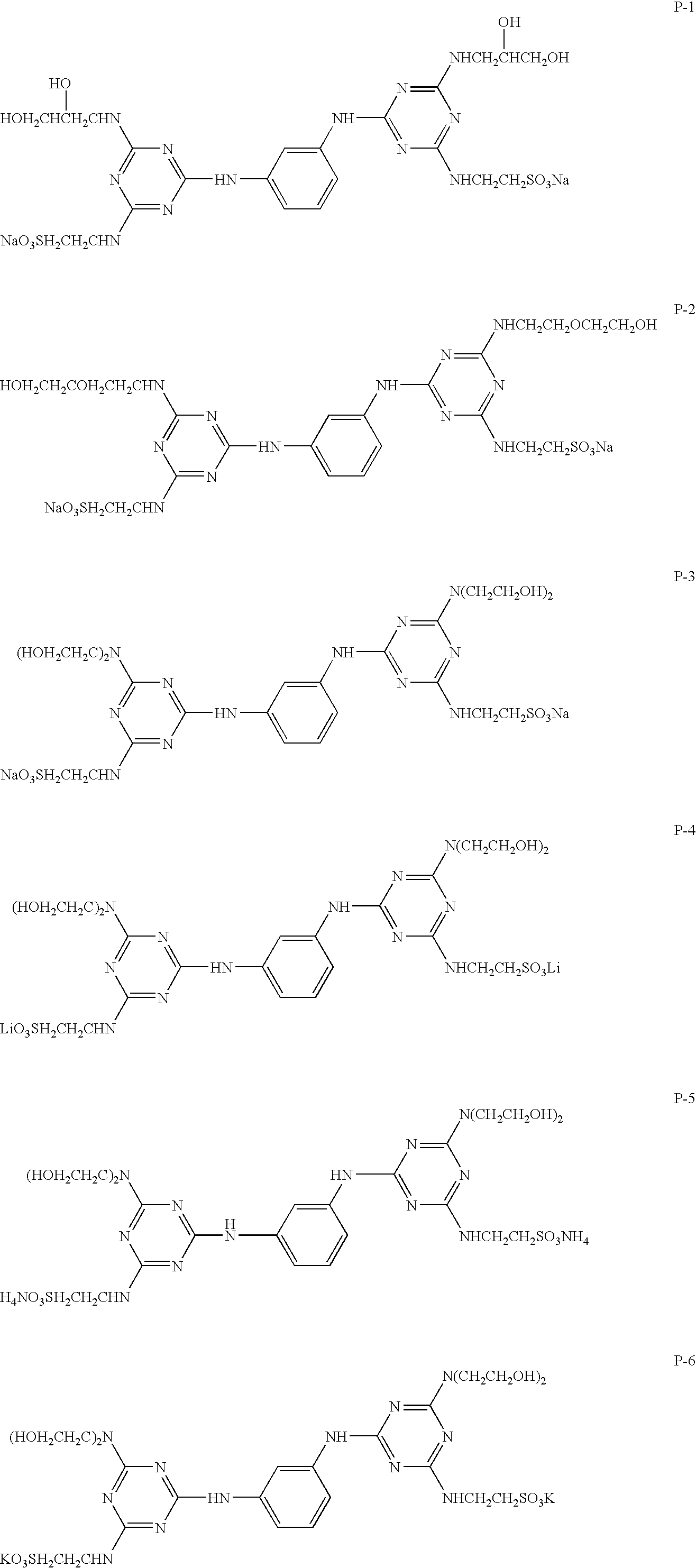
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
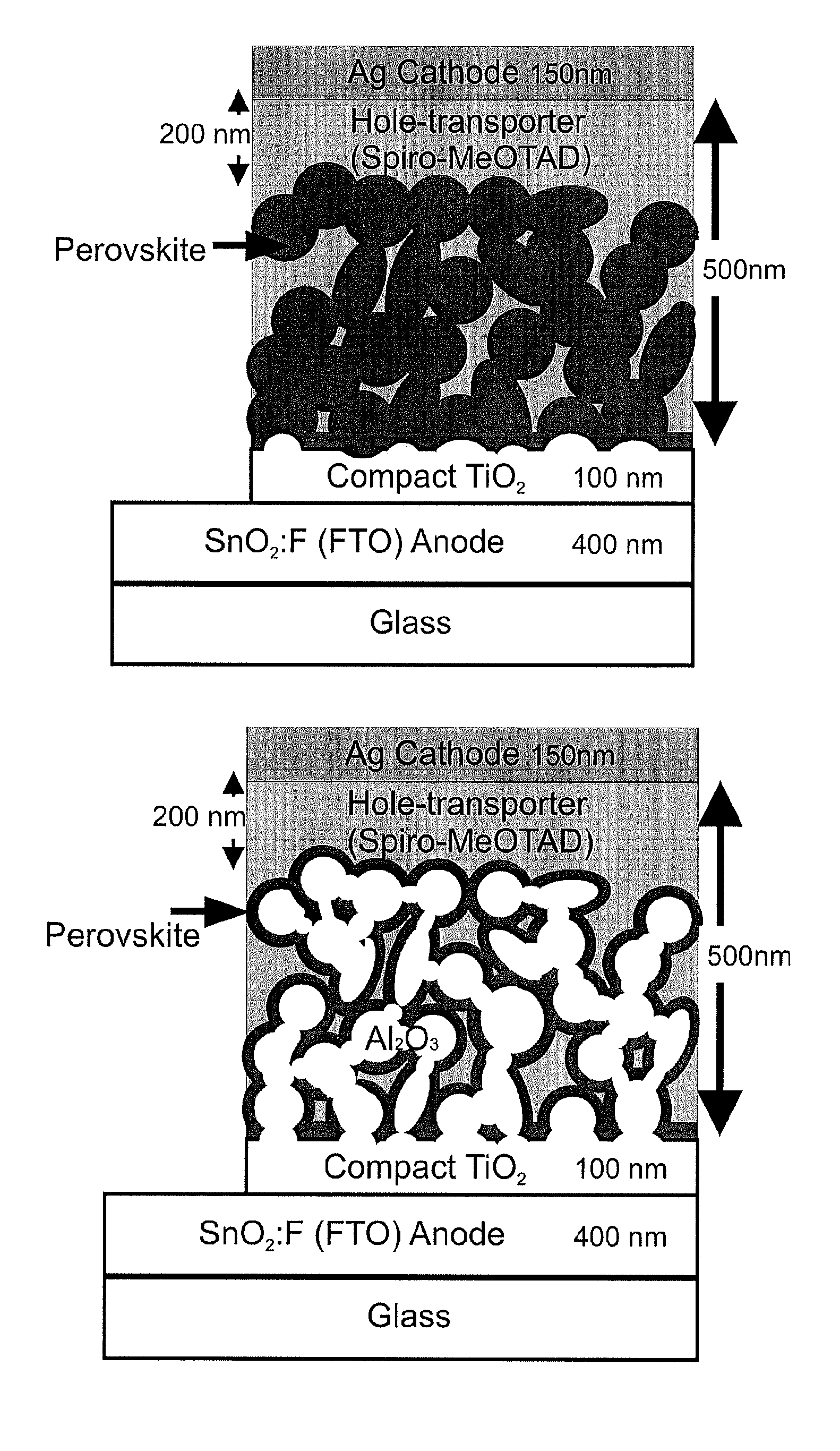
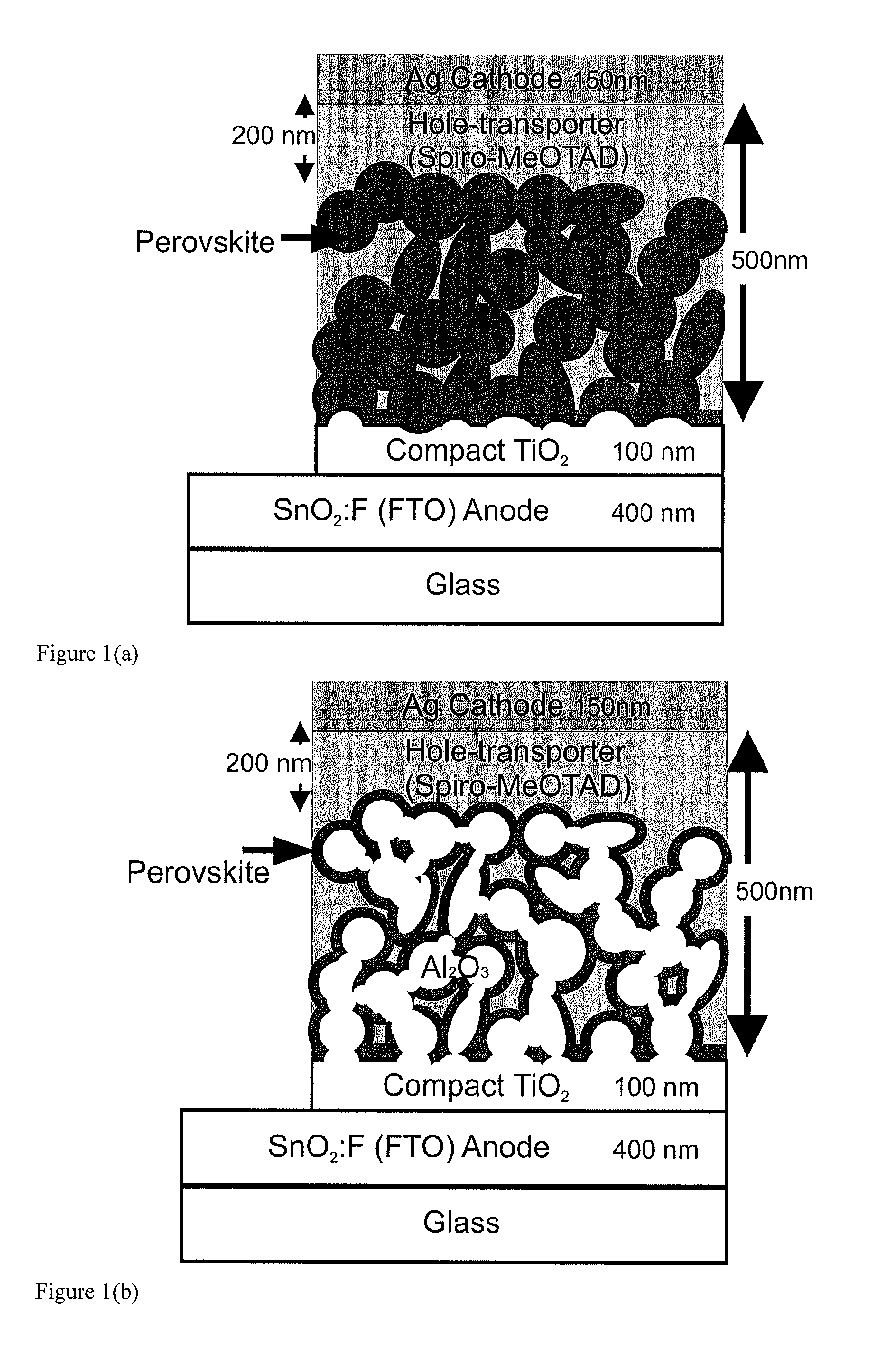
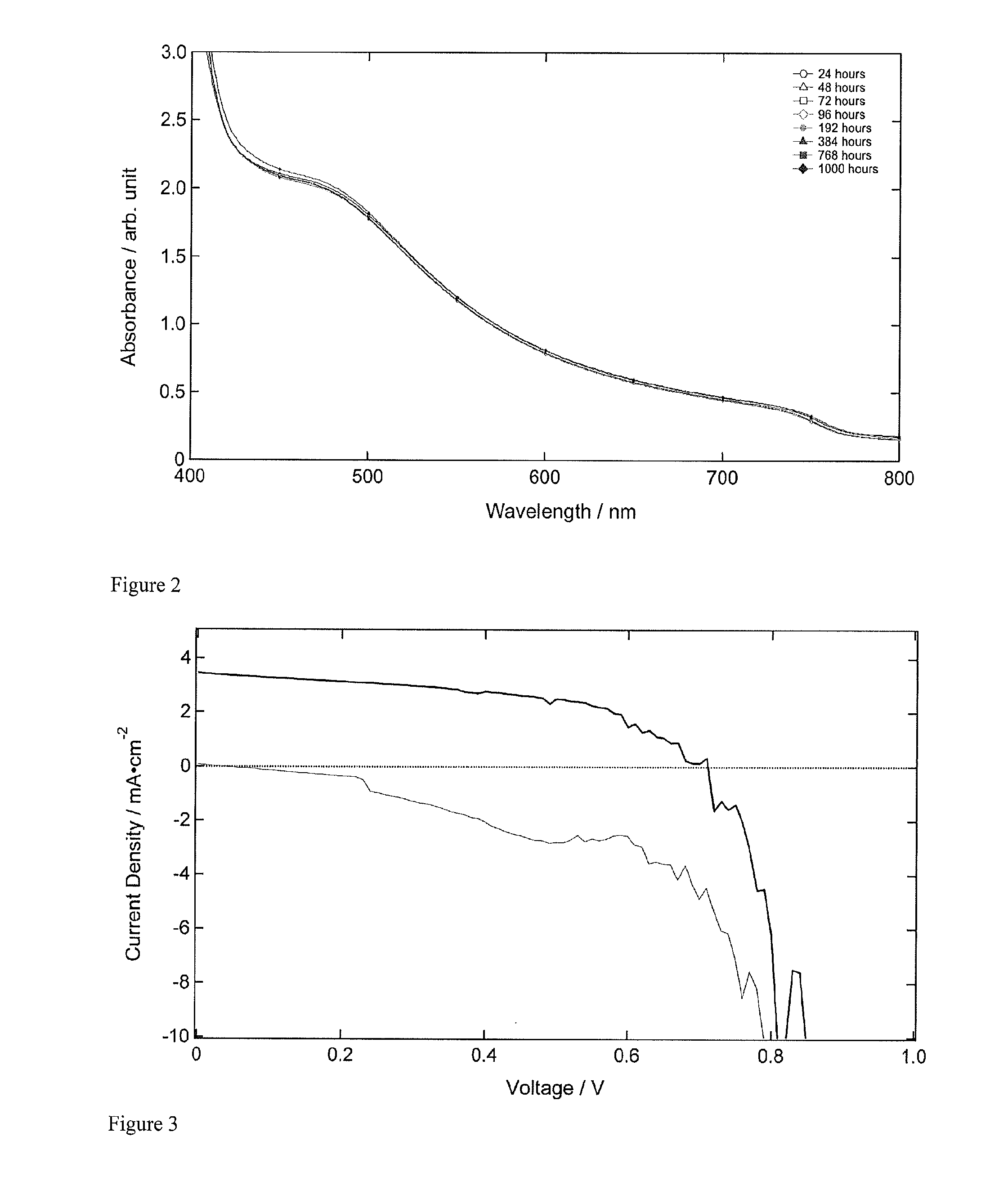
Optoelectronic device comprising perovskites
ActiveUS20150129034A1Excellent propertyHigh device efficiencyLight-sensitive devicesFinal product manufactureElectrical conductorSemiconductor
The invention provides an optoelectronic device comprising a porous material, which porous material comprises a semiconductor comprising a perovskite. The porous material may comprise a porous perovskite. Thus, the porous material may be a perovskite material which is itself porous. Additionally or alternatively, the porous material may comprise a porous dielectric scaffold material, such as alumina, and a coating disposed on a surface thereof, which coating comprises the semiconductor comprising the perovskite. Thus, in some embodiments the porosity arises from the dielectric scaffold rather than from the perovskite itself. The porous material is usually infiltrated by a charge transporting material such as a hole conductor, a liquid electrolyte, or an electron conductor. The invention further provides the use of the porous material as a semiconductor in an optoelectronic device. Further provided is the use of the porous material as a photosensitizing, semiconducting material in an optoelectronic device. The invention additionally provides the use of a layer comprising the porous material as a photoactive layer in an optoelectronic device. Further provided is a photoactive layer for an optoelectronic device, which photoactive layer comprises the porous material.
Owner:OXFORD UNIV INNOVATION LTD
Carbon fiber and method for producing the same
ActiveUS20110033705A1Improved mechanical propertyExcellent propertyMaterial nanotechnologyMonocomponent synthetic polymer artificial filamentCrystalliteMaterials science
A carbon fiber having a lattice spacing (d002) of 0.336 nm to 0.338 nm and a crystallite size (Lc002) of 50 nm to 150 nm as measured and evaluated by X-ray diffraction and a fiber diameter of 10 nm to 500 nm, the carbon fiber having no branched structure.
Owner:TEIJIN LTD +1
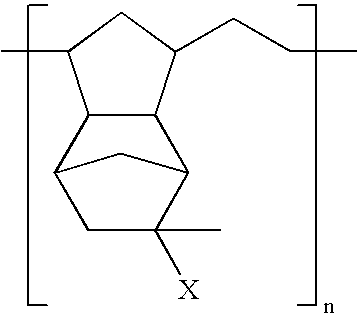
Solvent-free process for the preparation of mono-substituted fluorinated oxetane monomers
InactiveUS6037483AImprove hydrophobicityLower surface energyOrganic chemistry methodsPolyurea/polyurethane coatingsSide chainSolvent free
Owner:AMPAC FINE CHEM +1
Agonists of Guanylate Cyclase UseFul For The Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100093635A1Reduce inflammationPositive therapeutic effectAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Oligonucleotide analogues having modified intersubunit linkages and/or terminal groups
Oligonucleotide analogues comprising modified intersubunit linkages and / or modified 3′ and / or 5′-end groups are provided. The disclosed compounds are useful for the treatment of diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
Agonists of Guanylate Cyclase Useful for the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100069306A1Maintain good propertiesIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
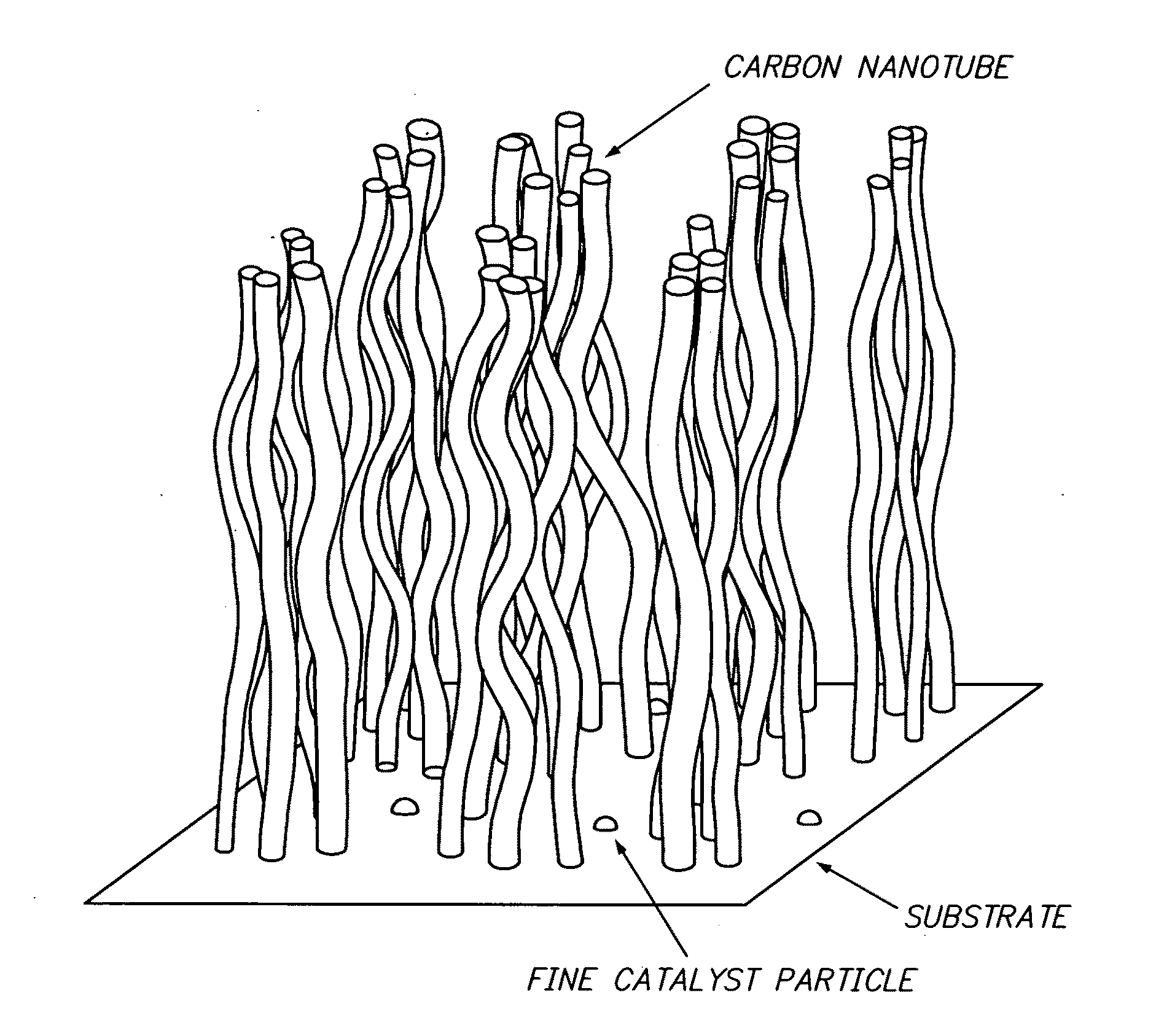
Aligned single-walled carbon nanotube aggregate, bulk aligned single-walled carbon nanotube aggregate, and powdered aligned single-walled carbon nanotube aggregate
ActiveUS20110008617A1Easy for density controlLow densityMaterial nanotechnologyLayered productsCarbon nanotubeAluminium oxide
This invention provides an aligned single-walled CNT aggregate comprising a substrate, fine particles of iron catalyst with a density of 1×1011 to 1×1014 / cm2 disposed on an alumina co-catalyst above the substrate, and a plurality of single-walled CNTs grown from the fine particles of the iron catalyst, in which the plurality of single-walled CNTs have a specific surface area of 600 m2 / g to 2600 m2 / g, and a weight density from 0.002 g / cm3 to 0.2 g / cm3, and the alignment degree which satisfies a few of specific conditions. This invention also provides a bulk aligned single-walled carbon nanotube aggregate and a powdered aligned single-walled carbon nanotube aggregate.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Implant for vessel ligature
InactiveUS20050266041A1Excellent propertyHighly physiological effectSurgeryBlood vesselsLanthanideOrthodontic ligature
The invention concerns inter alia an implant for vessel ligature, the implant which comprises an alloy, wherein the alloy is at least partially biodegradable, and wherein the alloy comprises: greater than 87% magnesium; from about 3% to about 6% yttrium; from about 1% to about 5% lanthanide; and a balance of about 0.0% to about 2%.
Owner:BIOTRONIK VI PATENT
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS7879802B2Excellent propertyIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
Owner:BAUSCH HEALTH IRELAND LTD
Thermoplastic cellulosic fiber blends as lost circulation materials
ActiveUS9453156B2Reduce circulation lossHigh densitySolid waste managementFlushingThermoplasticThermosetting polymer
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
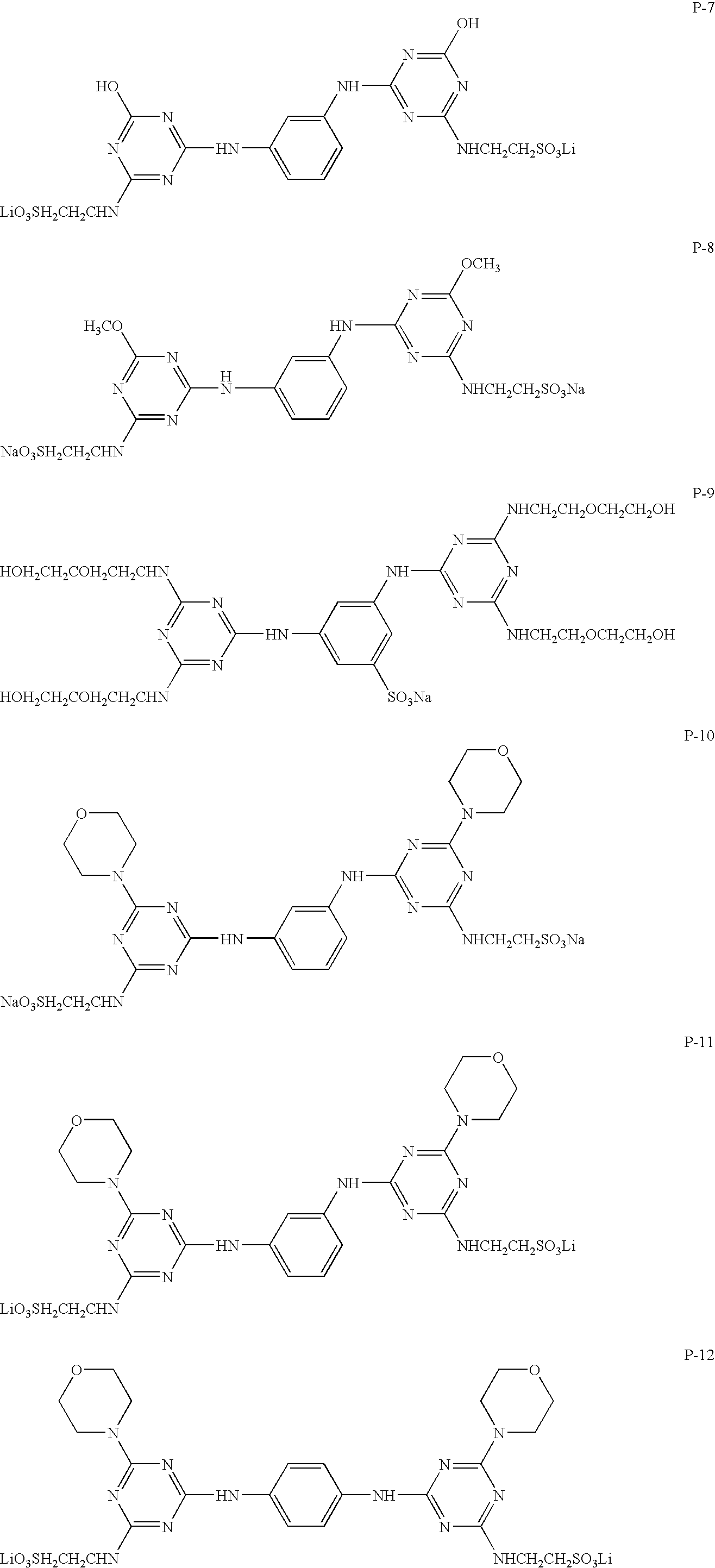
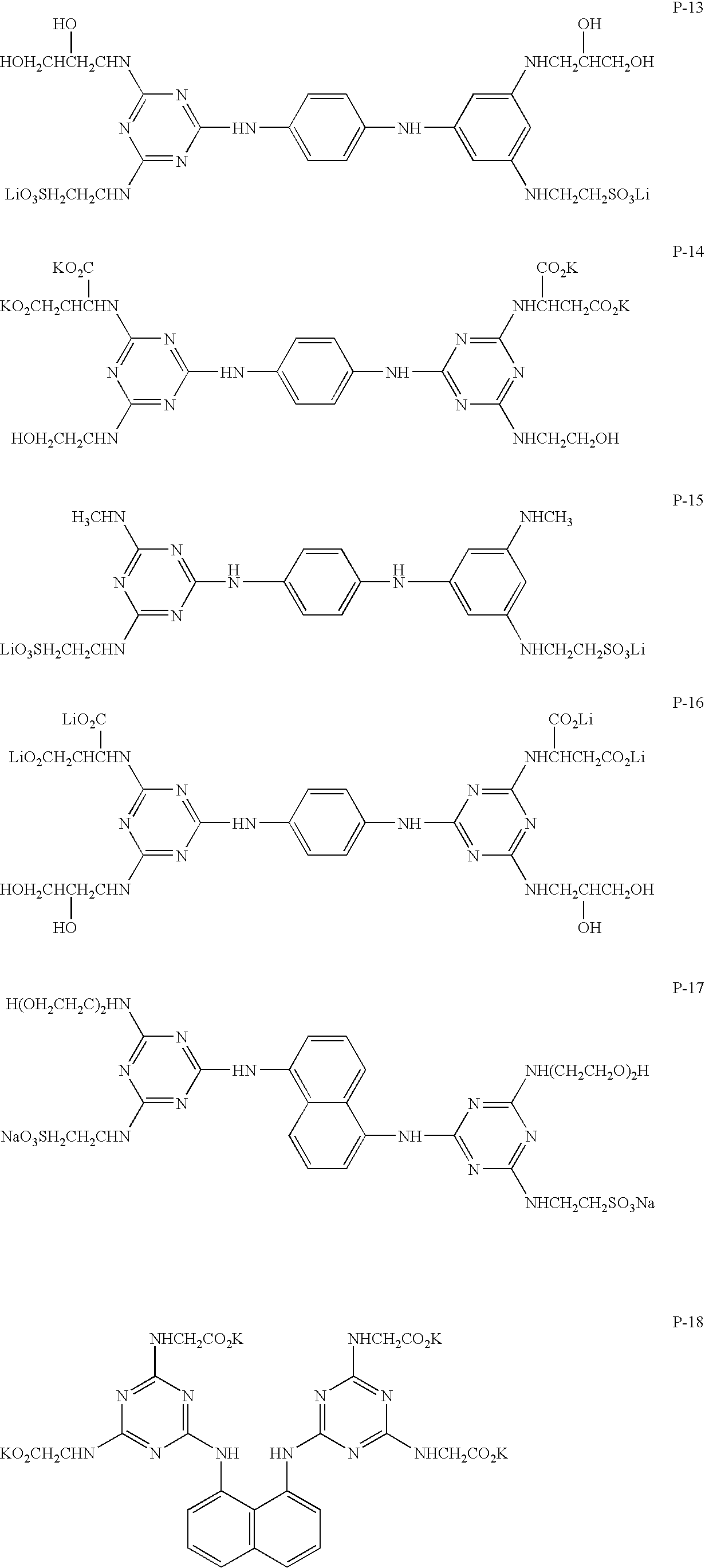
Recording medium, ink composition and recording method using the same
InactiveUS20050233097A1High resistanceExcellent propertyInksThermographyFluorescenceColor difference
A recording medium containing a substantially colorless compound in which at least 10 atoms having conjugate π electrons are present on a per-molecule basis, wherein it is especially preferable that the compound has at least two aromatic ring groups and / or shows no fluorescence. An ink composition used for printing in image form on media, with the composition containing a dye and controlling a hue change caused in the printed images over a period of one hour immediately after the printing to 5 or below in color-difference terms. And a method of recording images on media by printing an ink composition in image form, wherein the ink composition contains a dye and a hue change caused in the printed images over a period of one hour immediately after the printing is controlled to 5 or below in color-difference terms.
Owner:FUJIFILM HLDG CORP +1
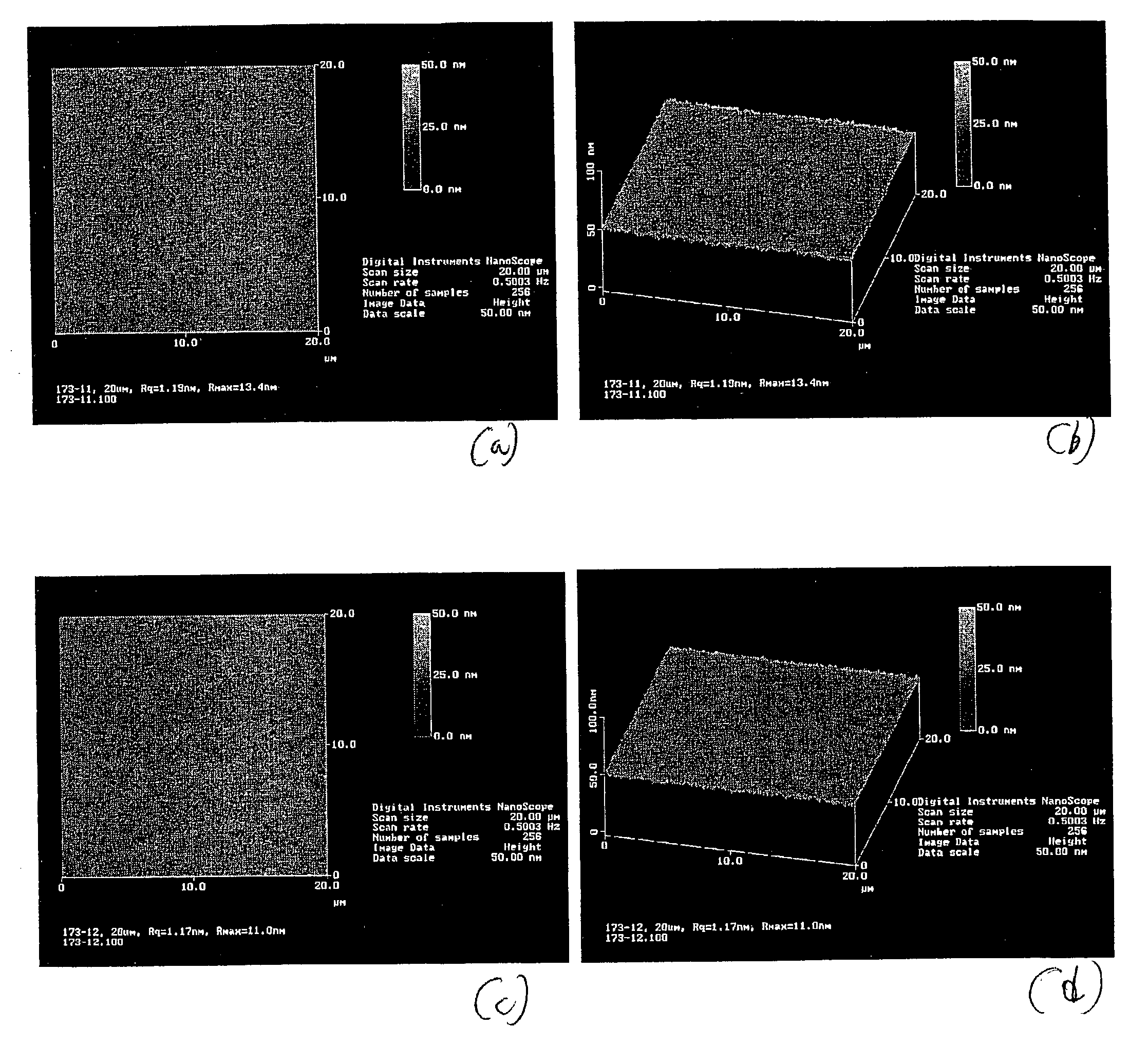
Transparent conductive film for flat panel displays
ActiveUS20040086717A1High transmittanceLow electric resistanceSolid-state devicesVacuum evaporation coatingTransmittanceIndium
Transparent conductive films for flat panel displays and methods for producing them are disclosed. In general, a method according to the present invention comprises: (1) providing a flexible plastic substrate; (2) depositing a multi-layered conductive metallic film on the flexible plastic substrate by a thin-film deposition technique to form a composite film, the multi-layered conductive metallic film comprising two layers of an alloy selected from the group consisting of indium cerium oxide (InCeO) and indium tin oxide (ITO) surrounding a layer of an alloy of silver, palladium, and copper (Ag / Pd / Cu); and (3) collecting the composite film in continuous rolls. Typically, the thin-film deposition technique is DC magnetron sputtering. Another aspect of the invention is a composite film produced by a method according to the present invention. Still another aspect of the invention is a composite film comprising a multilayered film as described above formed on a flexible plastic substrate, wherein the composite film has a combination of properties including: transmittance of at least 80% throughout the visible region; an electrical resistance of no greater than about 10 Omega / square; a root-mean-square roughness of no greater than about 2.5 nm; and an interlayer adhesion between the multi-layered metallic film and the remainder of the composite film that is sufficiently great to survive a 180° peel adhesion test.
Owner:XYLON LLC
Moisture curable sealer and adhesive composition
ActiveUS20050107499A1Low costExcellent propertyRoof covering using flexible materialsBituminous material adhesivesIsocyanatePliability
A low cost moisture curable sealer and adhesive composition containing a polymer having reactive silyl groups and a bituminous material and having many advantages over conventional moisture cure sealer compositions, including greater elastomeric properties, improved flexibility and pliability, lower durometer, faster and deeper cure, low temperature cure. The composition is also free of carcinogens such as coal tar, toxic isocyanates, and volatile solvents.
Owner:CHEMLINK
Copolymers and coprepolymers formed from mono-substituted fluorinated oxetane monomers and tetrahydrofuran
InactiveUS6380351B1Improve hydrophobicityLower surface energyOrganic chemistry methodsPolyurea/polyurethane coatingsSide chainButane
Owner:AEROJET GENERAL CORP +1
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8034782B2Excellent propertyIncrease resistanceAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Conductive Material For Connecting Part And Method For Manufacturing The Conductive Material
ActiveUS20080090096A1Excellent propertyLow coefficient of frictionHot-dipping/immersion processesElectrically conductive connectionsConductive materialsAlloy
There is provided a conductive material comprising a base material made up of a Cu strip, a Cu—Sn alloy covering layer formed over a surface of the base material, containing Cu in a range of 20 to 70 at.%, and having an average thickness in a range of 0.1 to 3.0 μm, and an Sn covering layer formed over the Cu—Sn alloy covering layer having an average thickness in a range of 0.2 to 5.0 μm, disposed in that order, such that portions of the Cu—Sn alloy covering layer are exposed the surface of the Sn covering layer, and a ratio of an exposed area of the Cu—Sn alloy covering layer to the surface of the Sn covering layer is in a range of 3 to 75%. The surface of the conductive material is subjected to a reflow process and preferably, an arithmetic mean roughness Ra of the surface of the material in at least one direction, is not less than 0.15 μm while the arithmetic mean roughness Ra thereof, in all directions, is not more than 3.0 μm and the average thickness of the Cu—Sn alloy covering layer is preferably not less than 0.2 μm. The conductive material is fabricated by a method whereby the surface of the base material is subjected to roughening treatment, an Ni plating layer, a Cu plating layer and an Sn plating layer are formed, as necessary, over the surface of the base material, and subsequently, a reflow process is applied.
Owner:KOBE STEEL LTD
Functionally-modified oligonucleotides and subunits thereof
ActiveUS20140330006A1Excellent propertyReduce deliveryAntibacterial agentsSugar derivativesDiseaseEnd-group
Functionally-modified oligonucleotide analogues comprising modified intersubunit linkages and / or modified 3′ and / or 5′-end groups are provided. The disclosed compounds are useful for the treatment of diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com