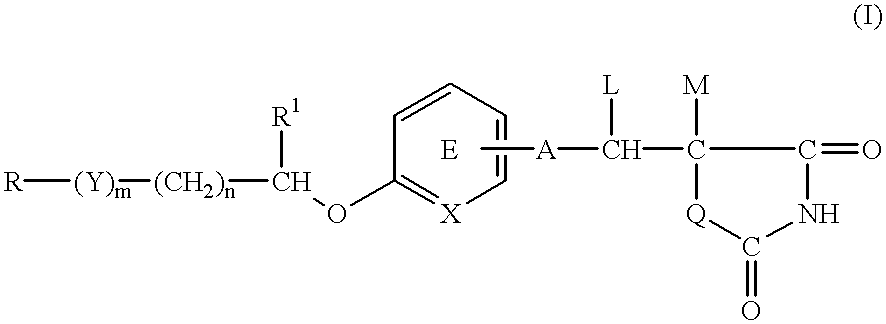
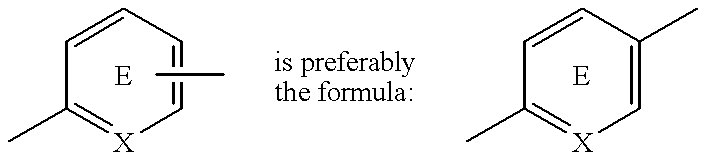Pharmaceutical composition for the treatment of diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 2
In the similar manner to Reference Example 1, 140000 tablets having the following composition and each containing 30 mg of pioglitazone were obtained.
reference example 3
In the similar manner to Reference Example 2, 140000 tablets having the following composition and each containing 45 mg of pioglitazone were obtained.
example 1
Effects of concomitant administration of pioglitazone hydrochloride and mazindol in noninsulin-dependent diabetic mellitus (NIDDM) patients were studied.
When pioglitazone hydrochloride (45 mg / day, oral administration) was concomitantly administered to a NIDDM patient [one sample(man); 44 years old; body weight 99.0 kg; fasting blood sugar 242.0 mg / dl; HbA.sub.1c 11.0%] under treatment with mazindol (1.0 mg / day, oral administration) over the period of 8 weeks, fasting blood sugar decreased by 70.0 mg / dl, HbA.sub.1c decreased by 2.00%, and body weight decreased by 1.00 kg.
When placebo (oral administration) was administered to NIDDM patients [55 samples (20 men and 35 women); 37 to 73 years old (57.9.+-.8.7 (means.+-.standard deviation) years old; body weight 59.8.+-.12.1 (means.+-.standard deviation of 54 samples) kg; fasting blood sugar 180.1 .+-.23.0 (means.+-.standard deviation) mg / dl; HbA.sub.1c 8.8.+-.1.3 (means.+-.standard deviation) %] over the period of 12 .+-.2 weeks, fasting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com