Nano-structural lipid carrier pharmaceutical composition and preparation method thereof
A technology of nanostructured lipids and compositions, applied in drug combinations, liposome delivery, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
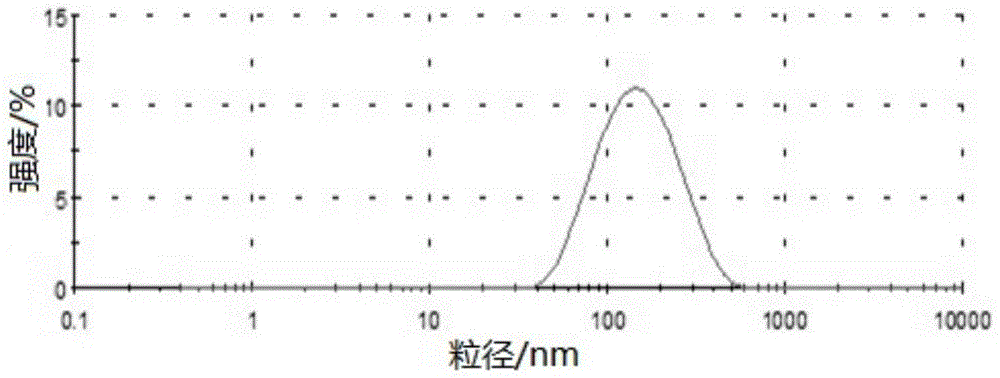
[0131] Embodiment 1 Preparation of bezafibrate nanostructured lipid carrier
[0132] Composition formula
[0133] Bezafibrate: 0.05g; solid lipid material glyceryl monostearate: 0.5g; liquid lipid material soybean oil: 10g; fat-soluble emulsifier lecithin: 10g; water-soluble emulsifier Poloxamer 188: 15g; distilled water: 65g.
[0134] The specific preparation method of the above-mentioned bezafibrate nanostructured lipid carrier is as follows:
[0135] Weigh the raw materials according to the weight ratio of the above components, add 10g of fat-soluble emulsifier lecithin and 15g of water-soluble emulsifier Poloxamer-188 into distilled water, heat in a water bath at 65°C and stir evenly to obtain a water phase.
[0136] Heat 0.5g of solid lipid material glyceryl monostearate and 10g of liquid lipid material soybean oil in a 65°C water bath and stir to obtain a liquid oil phase, then add 0.05g of bezafibrate into the liquid oil phase and heat to completely dissolved;
[01...
Embodiment 2
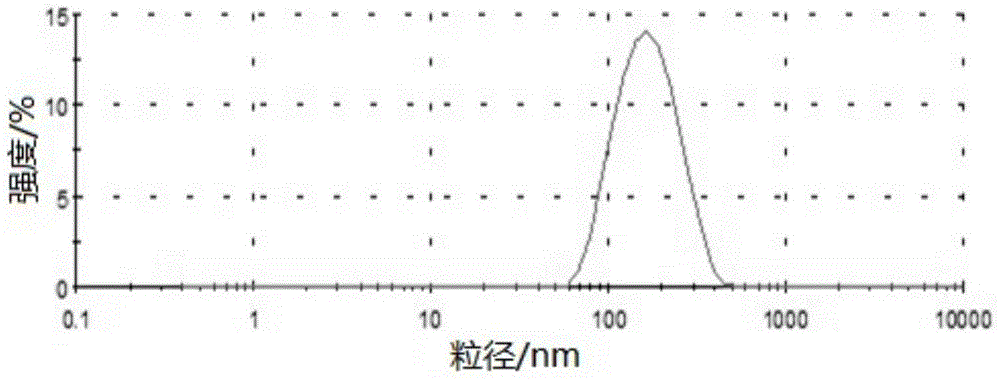
[0138] Preparation of Example 2 Bezafibrate Nanostructured Lipid Carrier
[0139] Composition formula
[0140] Bezafibrate: 0.05g; solid lipid material glyceryl monostearate: 0.3g; liquid lipid material soybean oil: 1g; fat-soluble emulsifier lecithin: 1g; water-soluble emulsifier Poloxamer 188: 1.5g; Phosphate buffer solution with a pH of 7.4 and a concentration of 50mmol / L: 46g.
[0141] The specific preparation method of the above-mentioned bezafibrate nanostructured lipid carrier is as follows:
[0142] Weigh the raw materials according to the weight ratio of the above components, add 1g of fat-soluble emulsifier lecithin and 1.5g of water-soluble emulsifier Poloxamer-188 into phosphate buffer, heat in a water bath at 65°C and stir evenly to obtain a water phase .
[0143] Heat 0.3g of solid lipid material glyceryl monostearate and 1g of liquid lipid material soybean oil in a 65°C water bath and stir evenly to obtain a liquid oil phase, then add 0.05g of bezafibrate int...
Embodiment 3
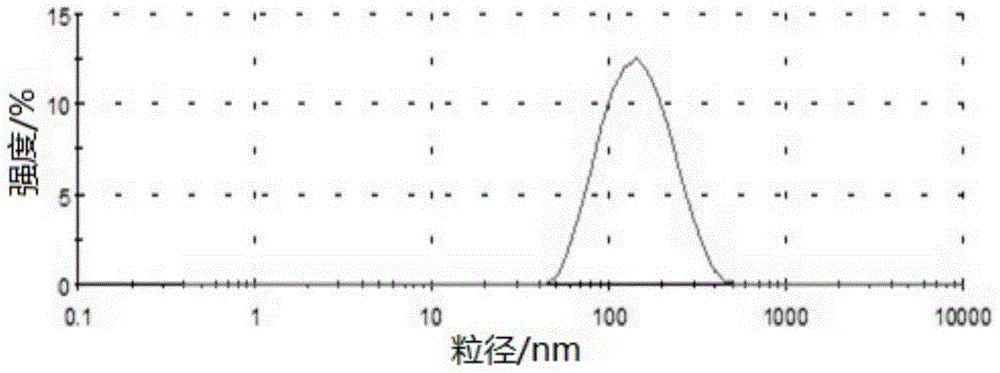
[0145] Example 3 Preparation of Bezafibrate Nanostructured Lipid Carrier
[0146] Composition formula
[0147] Bezafibrate: 0.1g; solid lipid material glyceryl monostearate: 1g; liquid lipid material soybean oil: 1g; fat-soluble emulsifier lecithin: 1g; water-soluble emulsifier Poloxamer 188: 3g ; Water for injection: 44g.
[0148] The specific preparation method of the above-mentioned bezafibrate nanostructured lipid carrier is as follows:
[0149] Weigh the raw materials according to the weight ratio of the above components, add 1g of fat-soluble emulsifier lecithin and 3g of water-soluble emulsifier poloxamer-188 into water for injection, heat in a water bath at 65°C and stir evenly to obtain a water phase.
[0150] Heat 1g of the solid lipid material glyceryl monostearate and 1g of the liquid lipid material soybean oil in a 65°C water bath and stir evenly to obtain a liquid oil phase, then add 0.1g of bezafibrate into the liquid oil phase, and heat until completely diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com