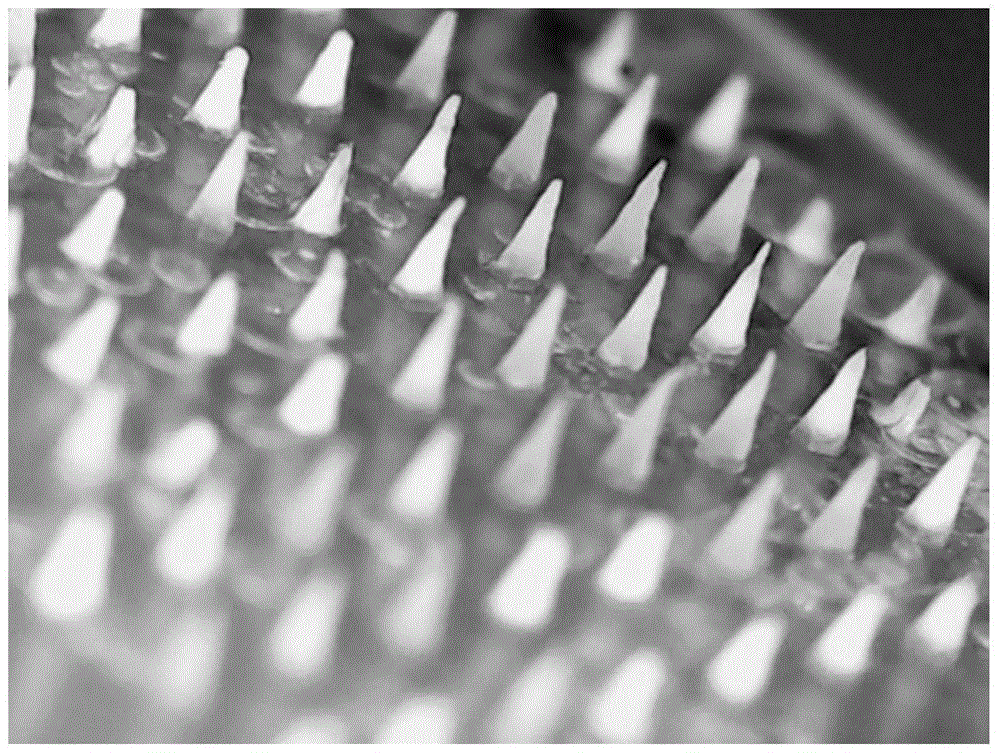
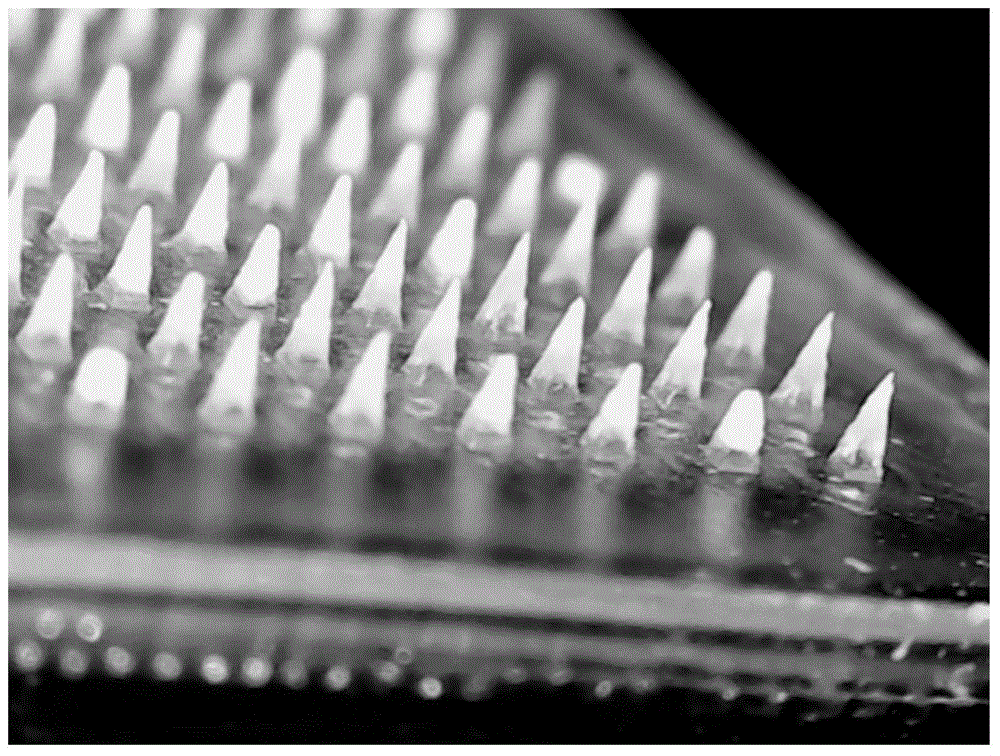
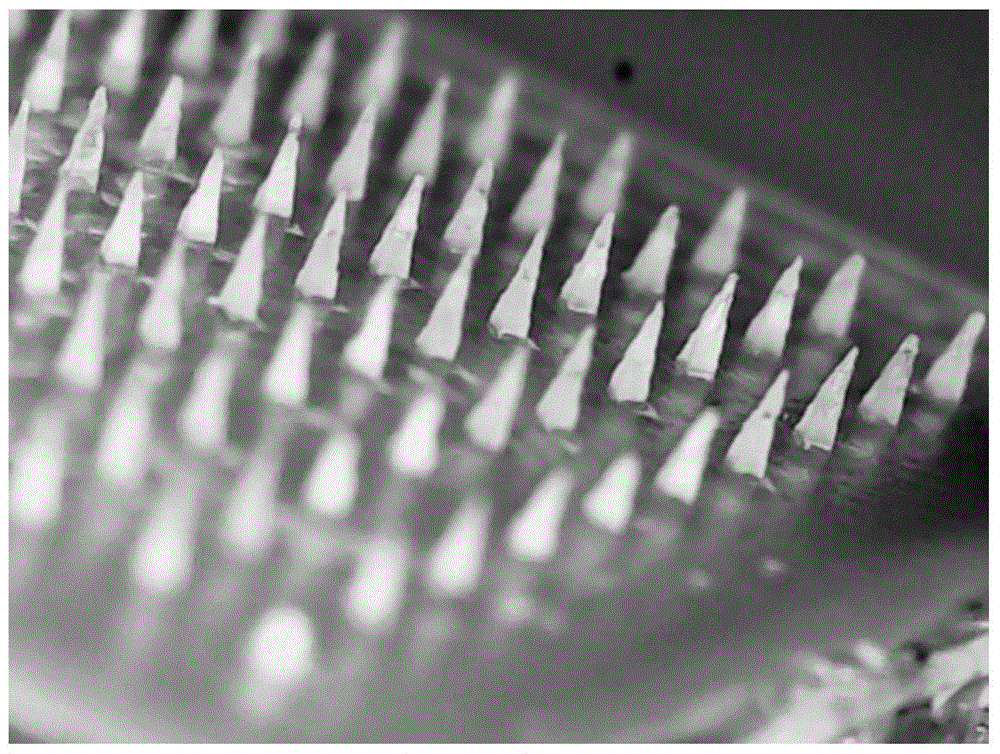
Temperature-sensitive soluble microneedle and preparation method thereof
A temperature-sensitive, soluble technology, used in pharmaceutical formulations, non-active medical preparations, drug delivery, etc., can solve problems such as increased skin irritation, slow dissolution, and unfavorable biological drug administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment provides a chitosan-β-sodium glycerophosphate-dextran temperature-sensitive soluble microneedle and a preparation method thereof, the preparation method comprising the following steps:
[0039] 1) Preparation of temperature-sensitive material solution
[0040] Take a certain amount of chitosan (degree of deacetylation greater than 95%), add 0.1mol / L hydrochloric acid solution, stir and dissolve to obtain a chitosan solution whose concentration of chitosan is 0.02g / ml; weigh a certain amount of β - Sodium glycerophosphate is dissolved in deionized water to obtain a β-sodium glycerophosphate solution whose concentration of β-sodium glycerophosphate is 1g / ml; get 1ml β-sodium glycerophosphate and add it dropwise to 5ml chitosan solution and stir for 30min to obtain the temperature Sensitive material solutions.
[0041] 2) Preparation of dextran solution
[0042] A certain amount of dextran (molecular weight 40000) was weighed and dissolved in deionized wa...
Embodiment 2
[0050] This embodiment provides a chitosan-β-sodium glycerophosphate-polyvinyl alcohol-dextran temperature-sensitive soluble microneedle and a preparation method thereof, the preparation method comprising the following steps:
[0051] 1) Preparation of temperature-sensitive material solution
[0052] Take a certain amount of chitosan (degree of deacetylation greater than 95%), add 0.1mol / L hydrochloric acid solution, stir and dissolve to obtain a chitosan solution whose concentration of chitosan is 0.02g / ml; weigh a certain amount of β - Sodium glycerophosphate is dissolved in deionized water to obtain a sodium β-glycerophosphate solution with a concentration of 1 g / ml of sodium β-glycerophosphate; a certain amount of polyvinyl alcohol is weighed, dissolved in deionized water, heated and stirred to obtain polyvinyl alcohol The concentration of vinyl alcohol is the polyvinyl alcohol solution of 0.11g / ml; 1ml chitosan solution and 1ml polyvinyl alcohol solution are stirred and m...
Embodiment 3
[0070] The soluble microneedles prepared in Examples 1-2 and Comparative Example 1 were tested for skin solubility. The specific method was as follows: press the soluble microneedles on the skin of the depilated rat for 2 minutes, respectively at 0 min, 2 min, and 5 min. , 10min, 20min, 30min, 60min, 120min to take out the microneedle from the skin, take pictures under the microscope to measure the remaining height of the needle tip, the results are as follows Figure 4 as well as Figure 5 shown.
[0071] The results show that: at 2 minutes, the needle tip remaining heights of Examples 1 and 2 were 69% and 62% of the original height, respectively, while the needle tip remaining height of Comparative Example 1 was 86% of the original height; The remaining needlepoint heights were 44% and 46% of the original height, respectively, while the remaining needlepoint heights of Comparative Example 1 were 58% of the original height; at 60 minutes, the remaining needlepoint heights of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com