Polypeptide K12-based transgenic vector and application thereof
A gene carrier and amino acid technology, applied in the field of medicine, can solve the problems of strong cytotoxicity, poor targeting of polyethyleneimine, and poor selection specificity, and achieve strong targeting, excellent transfection effect in cells and in vivo, and low toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation and functional verification of P407-PEI modified by polypeptide K12 (1)
[0041] 1. Preparation of P407-PEI
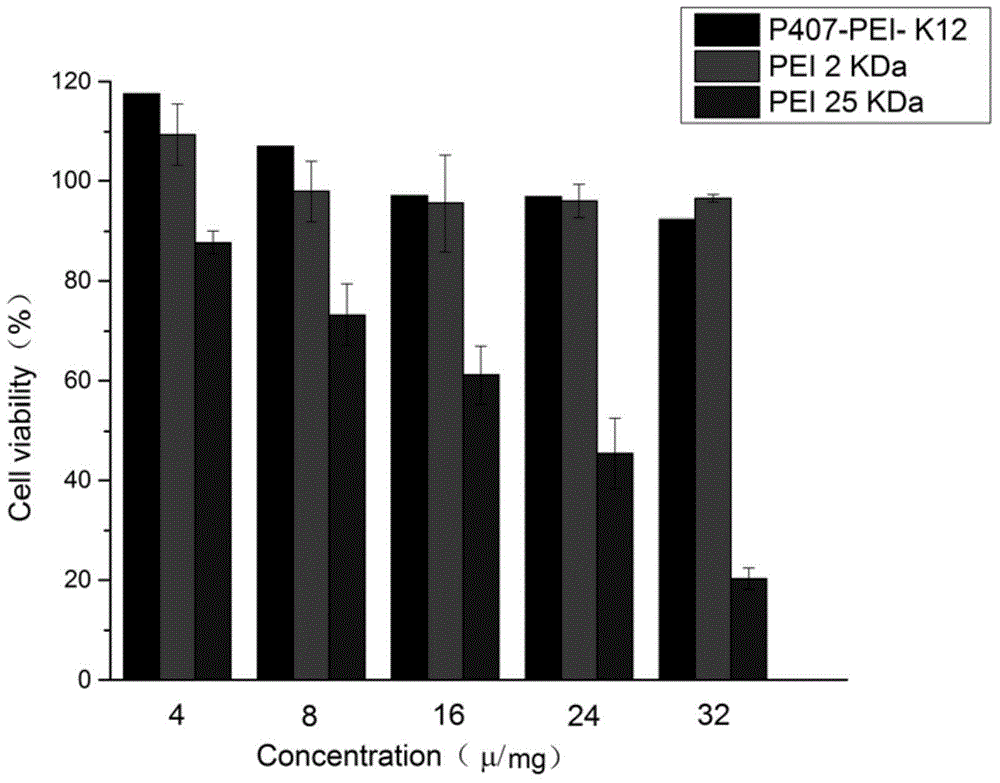
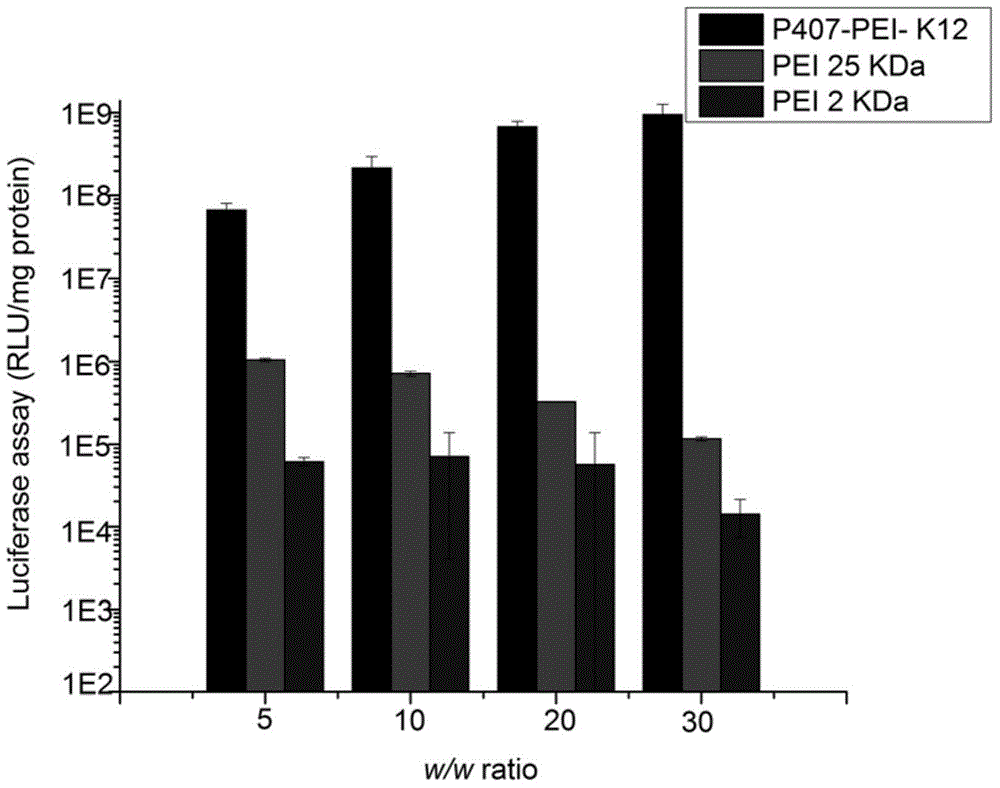
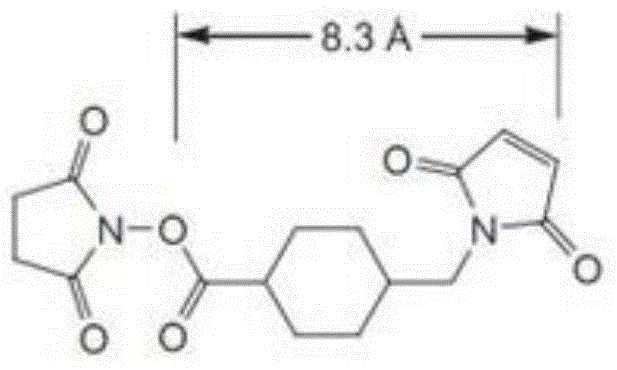
[0042] Weigh an appropriate amount of Poloxamer 407 (P407, 0.6 mmol) after water removal, and dissolve it in a mixed solution of anhydrous toluene and anhydrous dichloromethane, add 1.2 mmol of triphosgene, and magnetically stir the reaction overnight at room temperature. Remove the solvent by vacuum rotary evaporation, then dissolve it with an appropriate amount of anhydrous toluene and anhydrous dichloromethane, then add 2.0mmol of N-hydroxysuccinimide, and under magnetic stirring, add 2.0mmol of anhydrous triethylamine to the reaction solution dropwise , Continue to stir the reaction for about 4h. After the reaction is complete, the reaction solution is filtered and the solvent is removed by vacuum rotary evaporation again. The resulting residue is dissolved in ethyl acetate. The supernatant is taken after high-speed centrifugation and rotar...
Embodiment 2
[0060] Example 2 Preparation and functional verification of P407-PEI modified by polypeptide K12 (2)
[0061] The preparation and in vitro transfection experiment procedures of P407-PEI-K12 are the same as in Example 1, except that the molecular weight of PEI in this example is 70KDa, and the molar ratio of P407 to PEI used in the preparation of P407-PEI is 1:1. The molar ratio of peptide K12 to P407-PEI used in P407-PEI-K12 is 5:1. The results of in vivo transfection experiments showed that the expression intensity of P407-PEI-K12 fluorescein was much higher than that of P123-PEI-R11, and the expression intensity of P123-PEI-R11 in the heart, liver, spleen, lung, and kidney was 12, 13, 12, 11, 11 times.
Embodiment 3
[0062] Example 3 Preparation and functional verification of P407-PEI modified by polypeptide K12 (3)
[0063] The preparation and in vitro transfection experiment procedures of P407-PEI-K12 are the same as in Example 1, except that the molecular weight of PEI in this example is 4KDa, and the molar ratio of P407 to PEI used when preparing P407-PEI is 1:5. The molar ratio of peptide K12 to P407-PEI used in P407-PEI-K12 is 1:1. The results of in vivo transfection experiments showed that the expression intensity of P407-PEI-K12 fluorescein was much higher than that of P123-PEI-R11, and the expression intensity of P123-PEI-R11 in the heart, liver, spleen, lung, and kidney were 13, 14, 11, 12, 12 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com