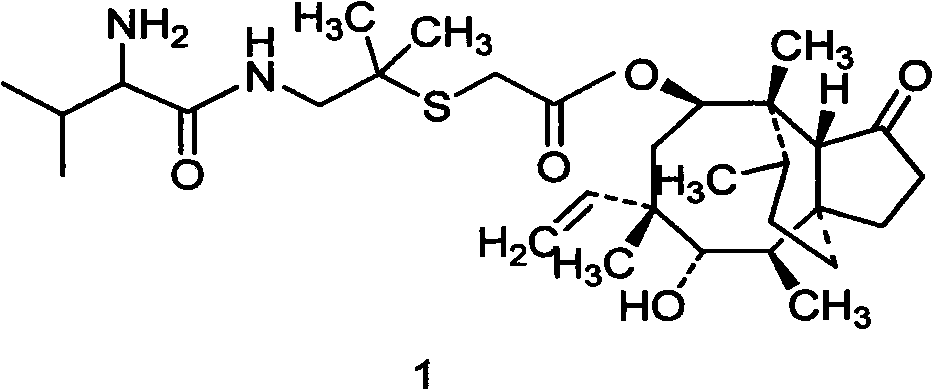
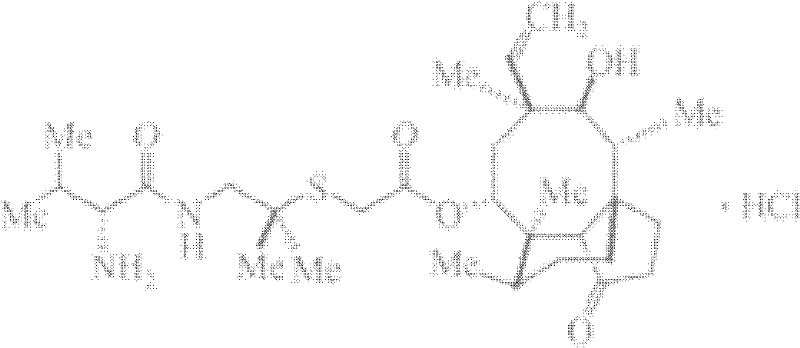
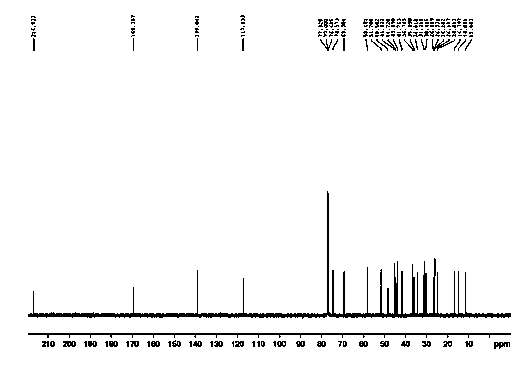
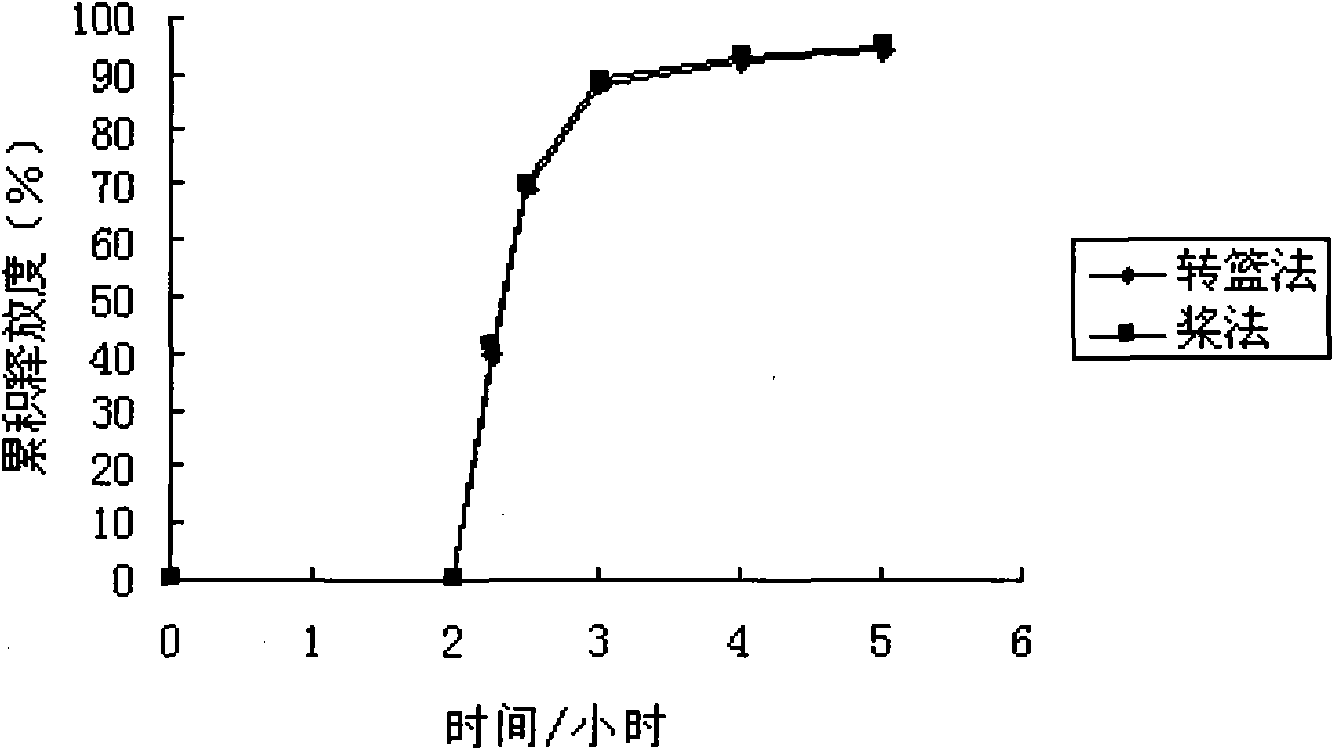
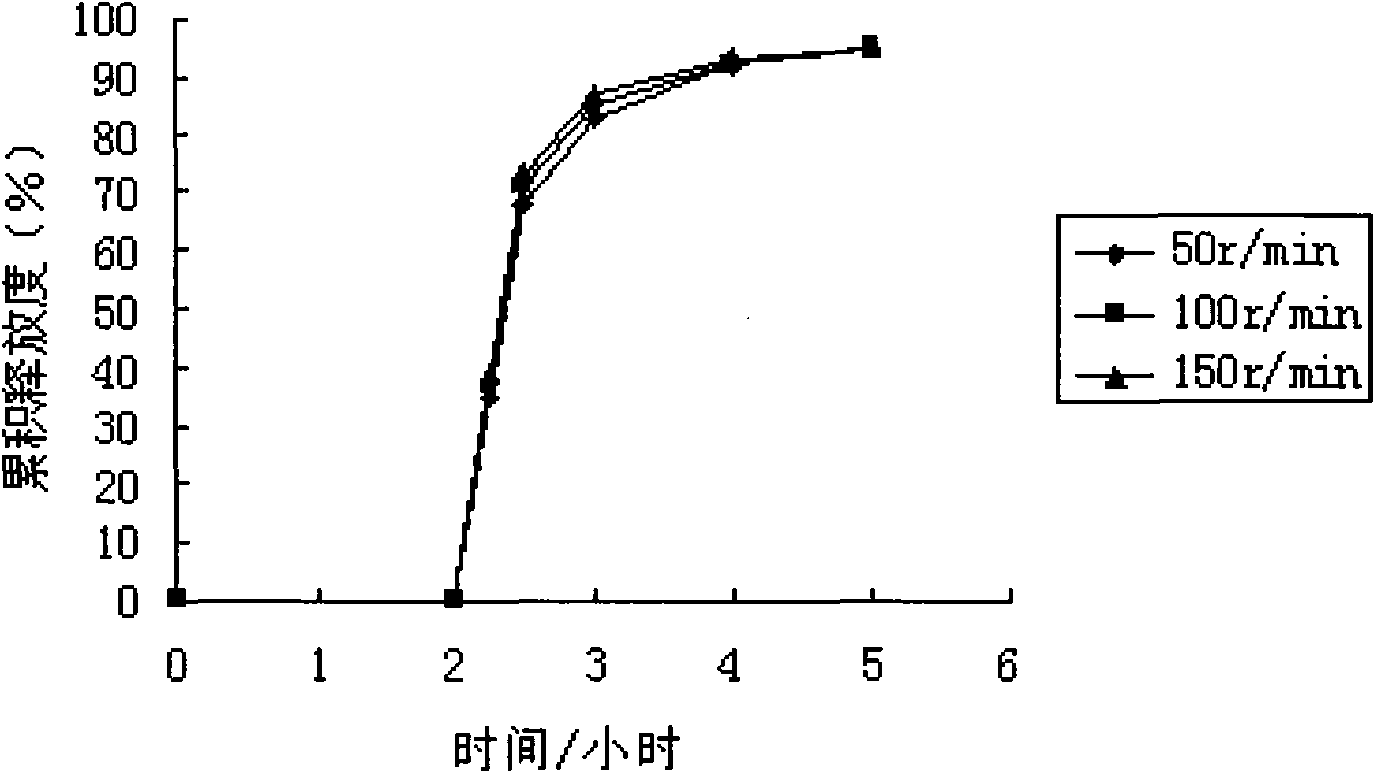
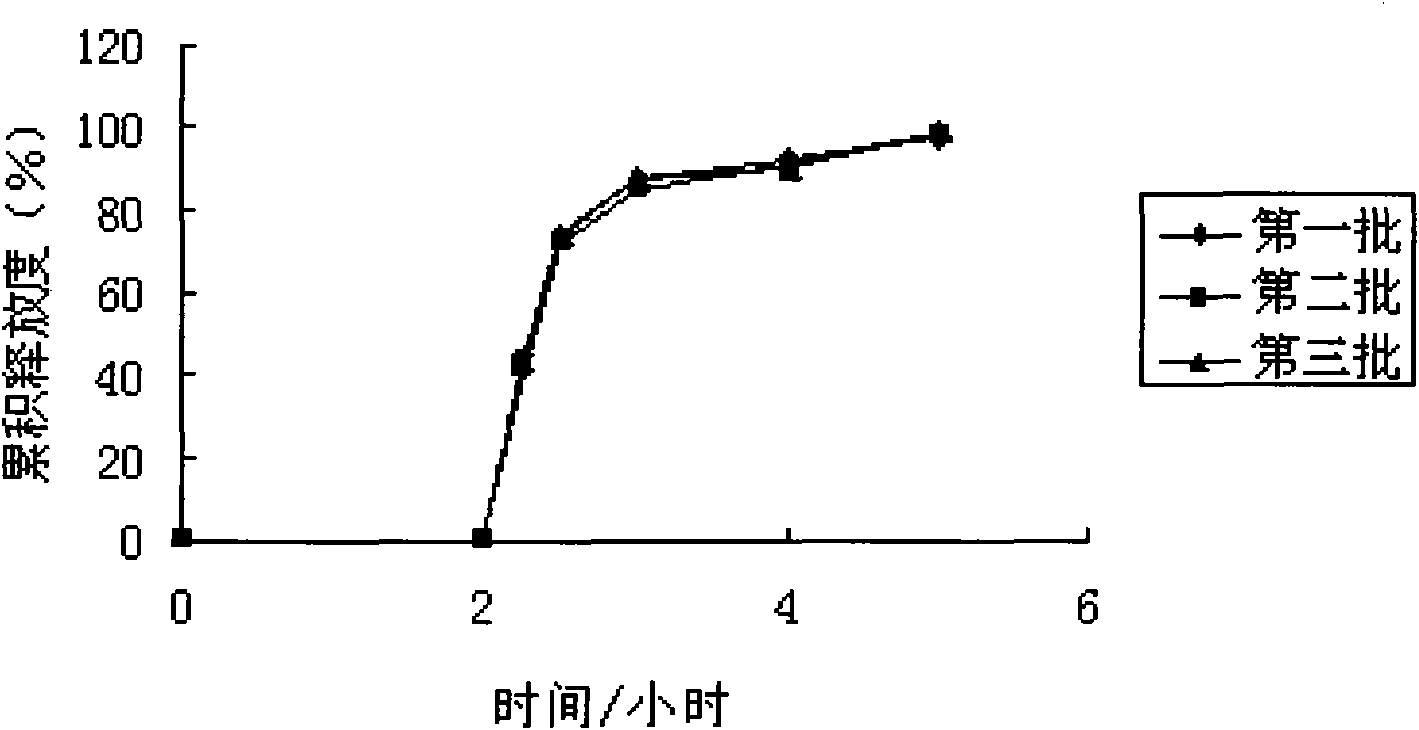
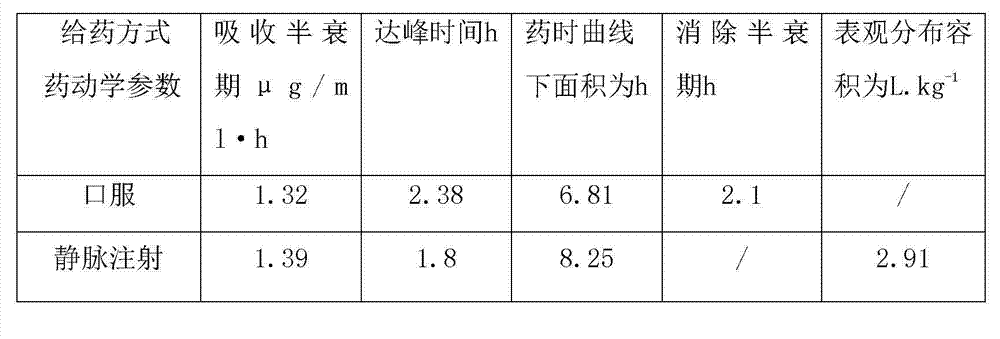
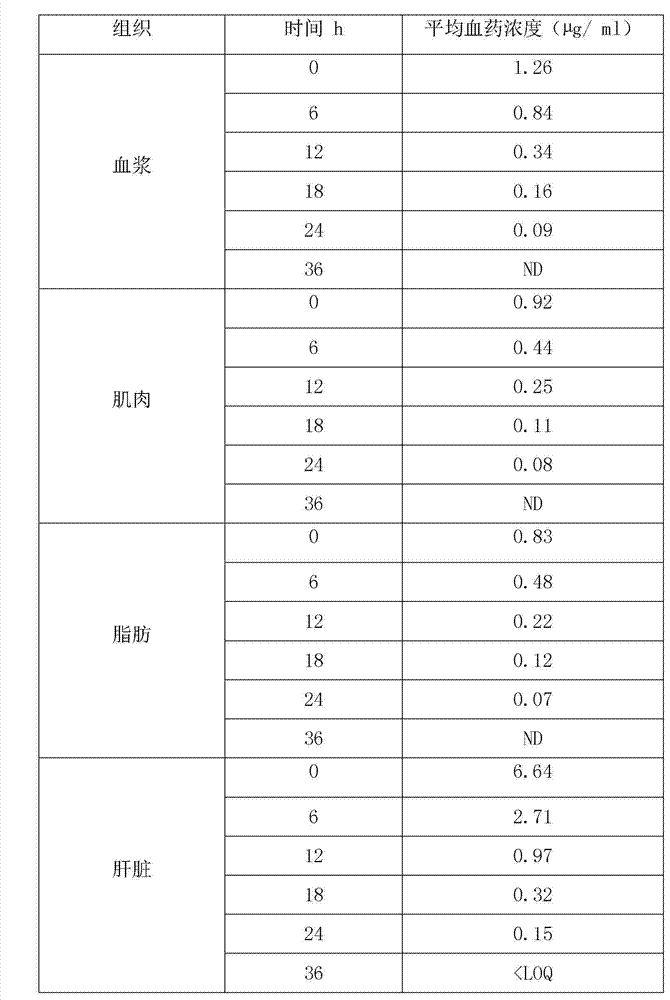
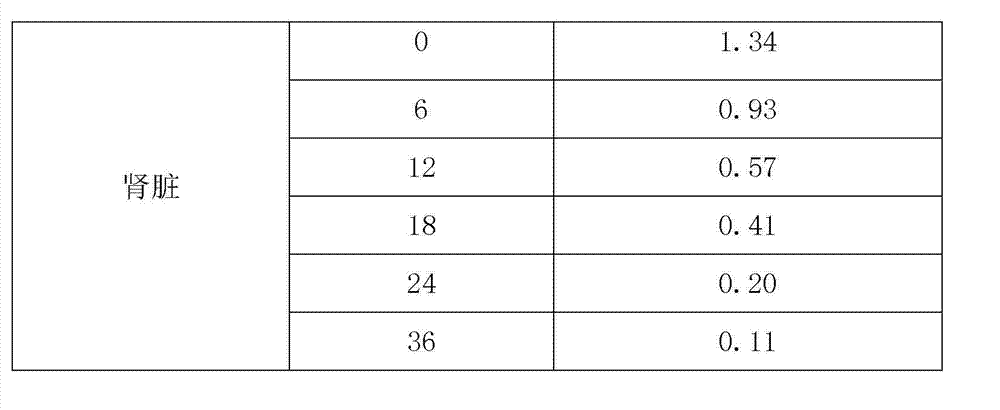
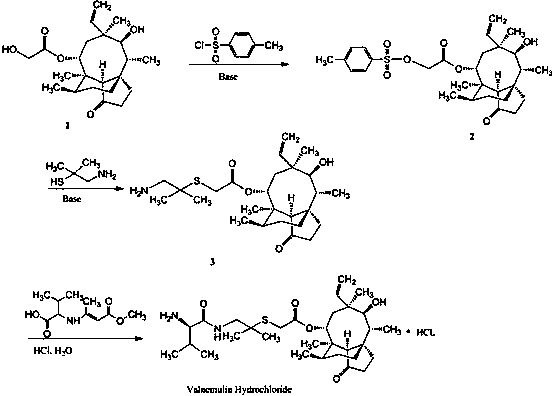
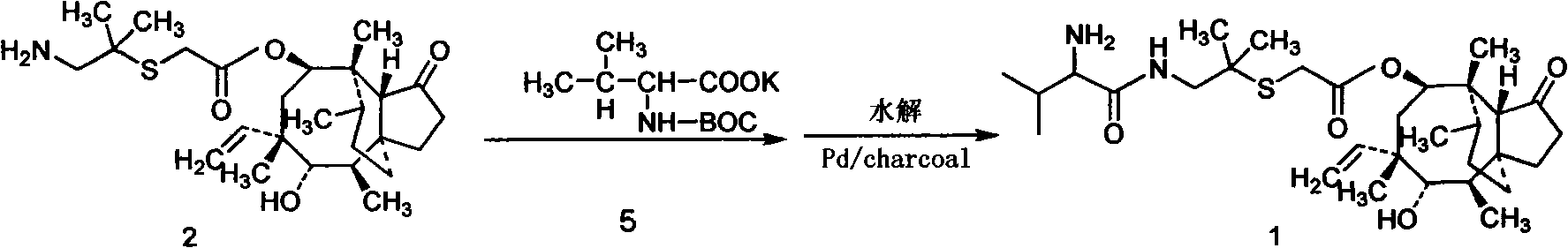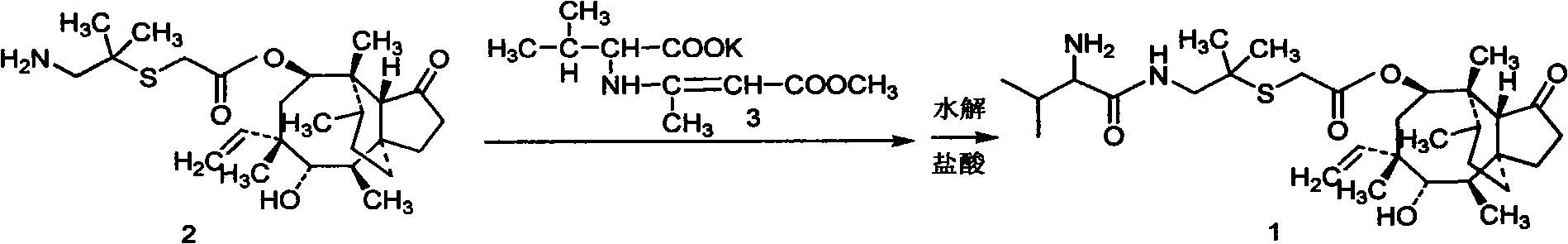Patents
Literature
59 results about "Valnemulin Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
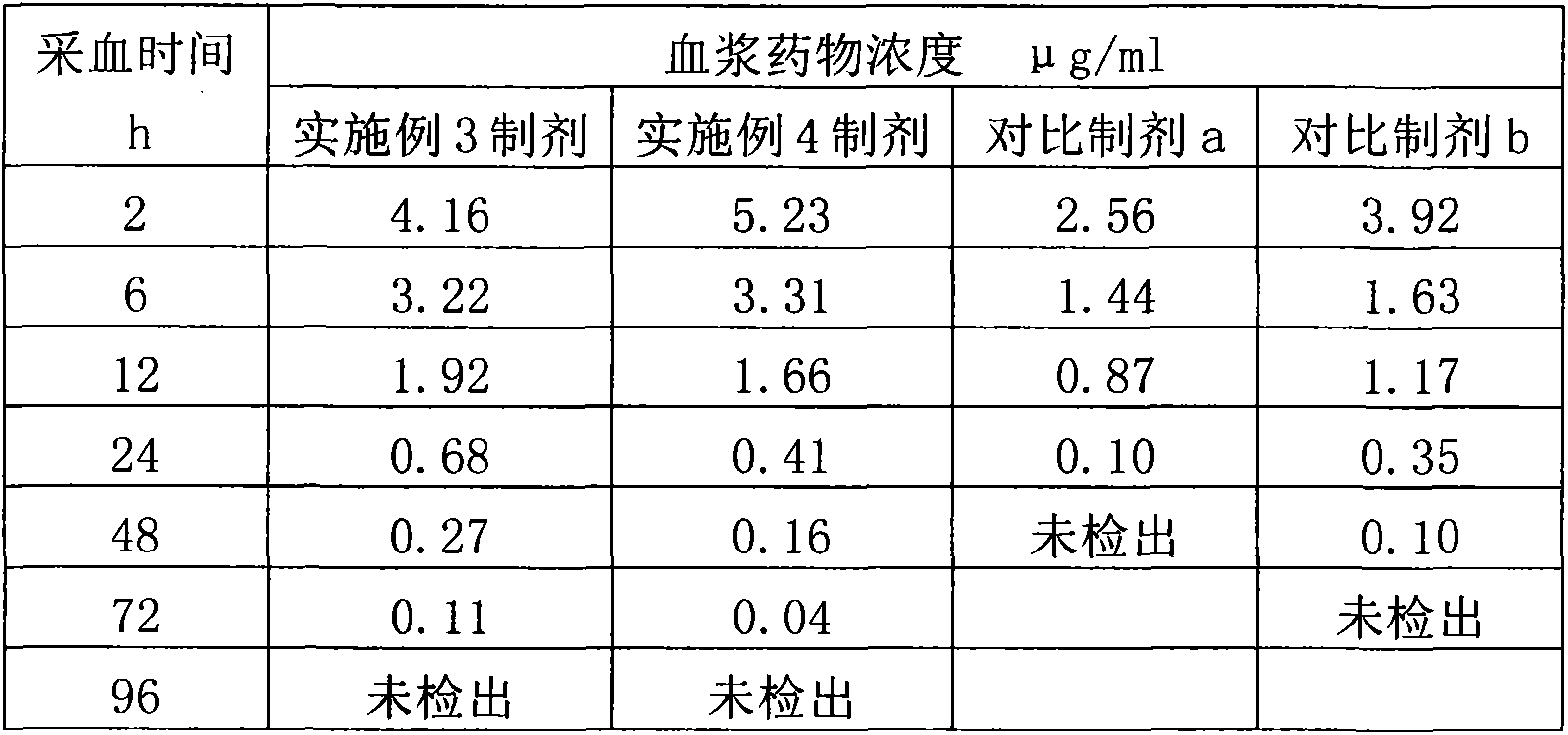
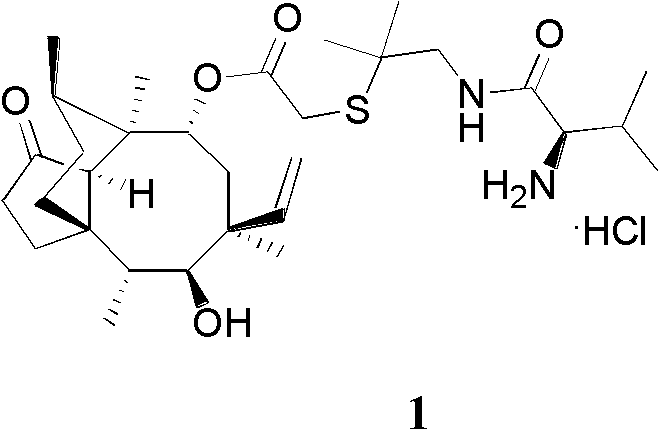
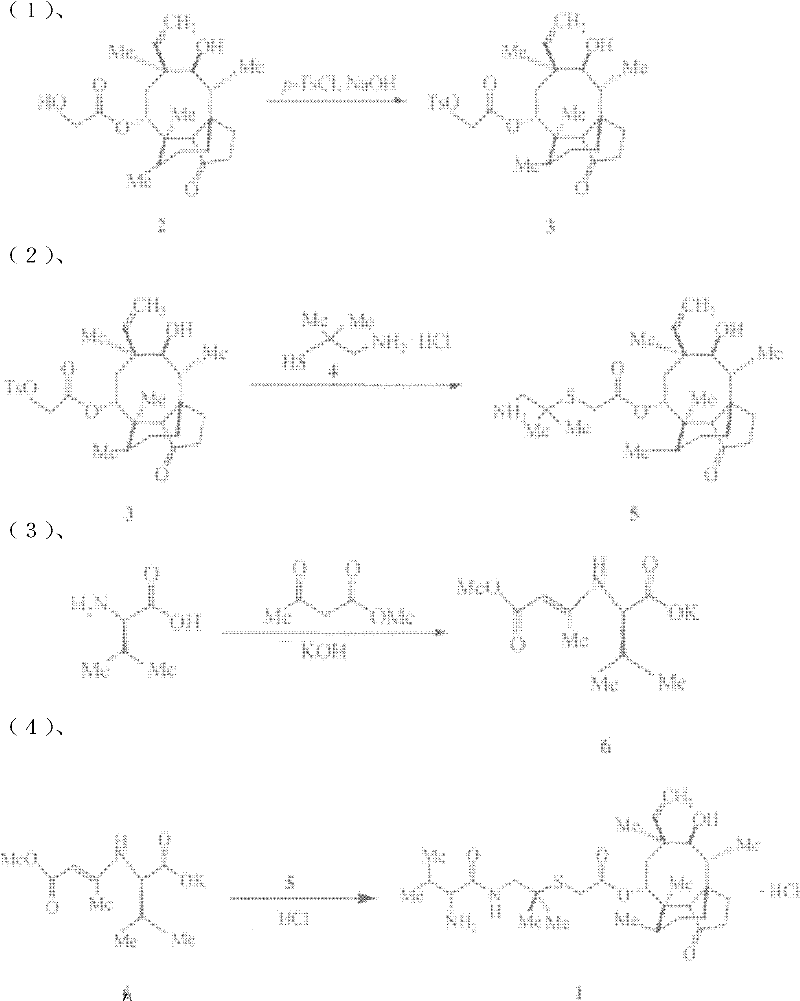
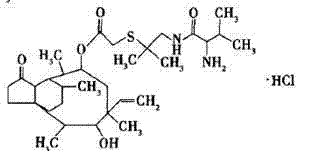
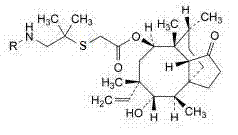
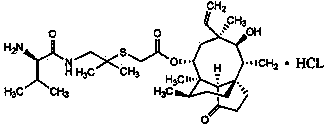
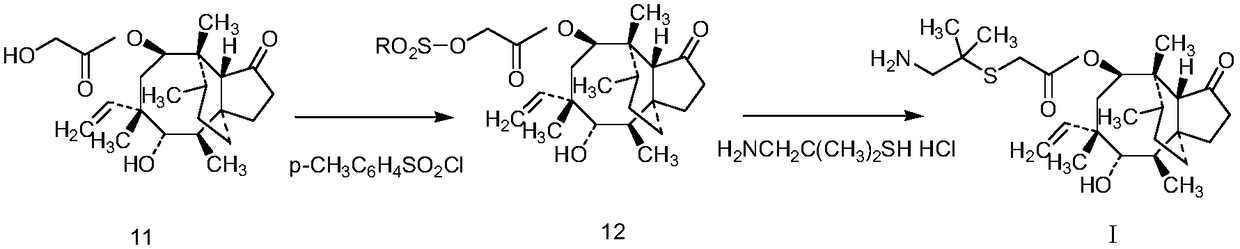
Crystallization method of valnemulin hydrochloride
ActiveCN102225906ASimple and fast operationSuitable for mass productionOrganic chemistryOrganic compound preparationOrganic solventValnemulin Hydrochloride
The invention mainly discloses a crystallization method of valnemulin hydrochloride. The method comprises the following steps: dissolving valnemulin in an organic solvent; adding a certain amount of dried hydrogen chloride gas or hydrogen chloride organic solution; separating out crystals; and collecting the obtained crystals, and drying so as to obtain valnemulin hydrochloride. The method is convenient for operation, is suitable for industrial production on large scale, has low cost and can be used for purifying the product. The chromatograph content of the valnemulin hydrochloride obtained by the method in the invention is improved by 1-5% as compared with the chromatograph content of the corresponding valnemulin.
Owner:ZHEJIANG GUOBANG PHARMA
Oily injection containing valnemulin hydrochloride/poloxamer 407
ActiveCN103705454AReduce dosageGood sustained release effectAntibacterial agentsSolution deliveryValnemulin HydrochlorideBiocompatibility Testing
The invention discloses an oily long-acting injection containing valnemulin hydrochloride / poloxamer 407 drug-loading particles, which is prepared by combining the valnemulin hydrochloride with poloxamer 407 into drug-loading particles and further dispersing the drug-loading particles into an oily medium and grinding. Hydroxypropyl methyl cellulose or high-substituted hydroxypropyl cellulose also can be added into the drug-loading particles, and the sustained-release effect of the preparation is obviously enhanced. The preparation process of the injection is simple, the drug release is uniform, the biocompatibility is good, irreversible damage to the tissue of the injection part is avoided, and the adopted sustained-release carrier can be degraded and excreted.
Owner:河北威远药业有限公司
Valnemulin hydrochloride emulsion for animals and preparation method thereof
InactiveCN101947203ARapid distribution in the bodyStrong targetingAntibacterial agentsDigestive systemMycoplasma penetransValnemulin Hydrochloride
The invention discloses valnemulin hydrochloride emulsion for animals. Each 100ml of emulsion mainly comprises the following components by weight: 5 to 10g of valnemulin hydrochloride, 1 to 5g of emulsifier, 20 to 40g of oil for injection, 5 to 15g of ethanol and the balance of water for injection. The valnemulin hydrochloride emulsion for animals has the advantages of quick in vivo distribution and good target, can reduce the medicament residue in vivo and the toxic or side effect on the animals, and also can mask the unpleasant odor of the medicament, and improve the stability of the medicament. The valnemulin hydrochloride emulsion for animals can effectively treat gram-positive bacteria and mycoplasma infection, particularly swine dysentery and porcine enzootic pneumonia, and can reduce the using amount of the medicament and avoid medicament tolerance.
Owner:ZHENGZHOU HOUYI PHARMA
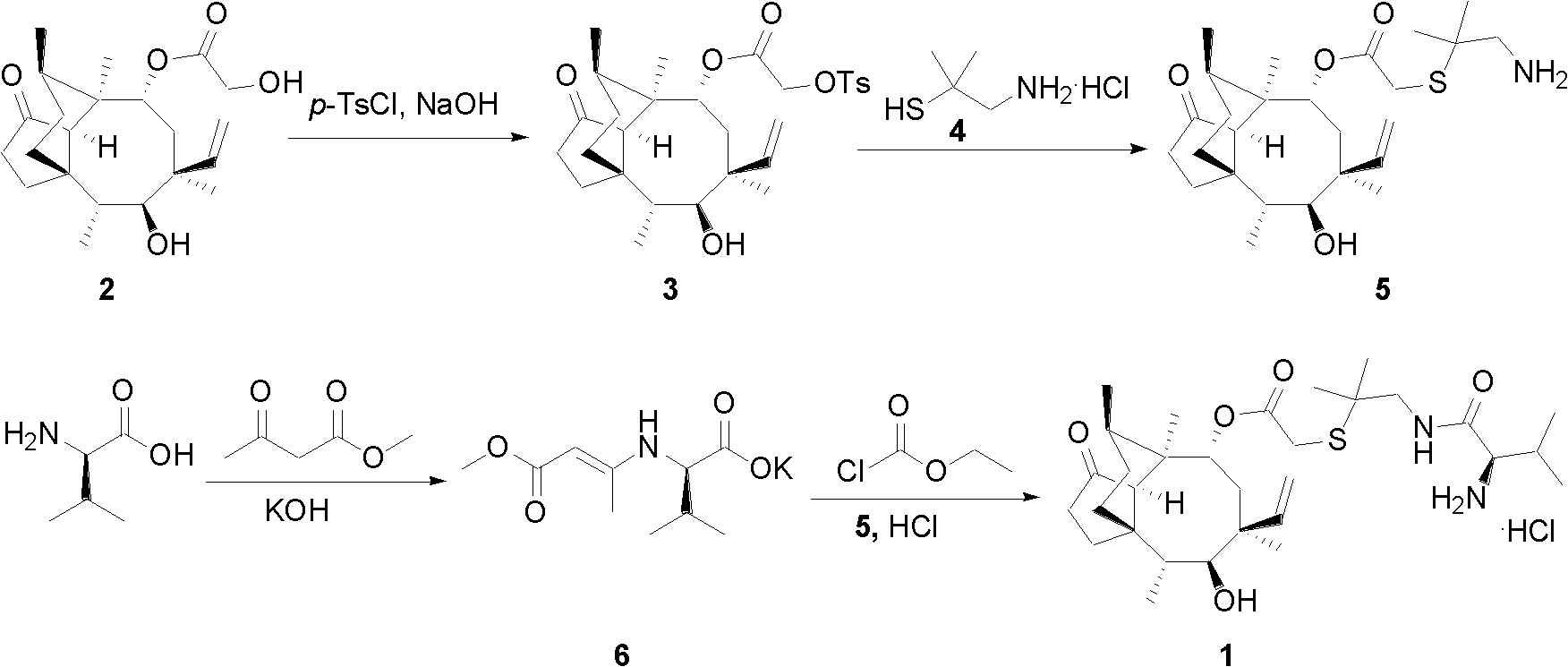
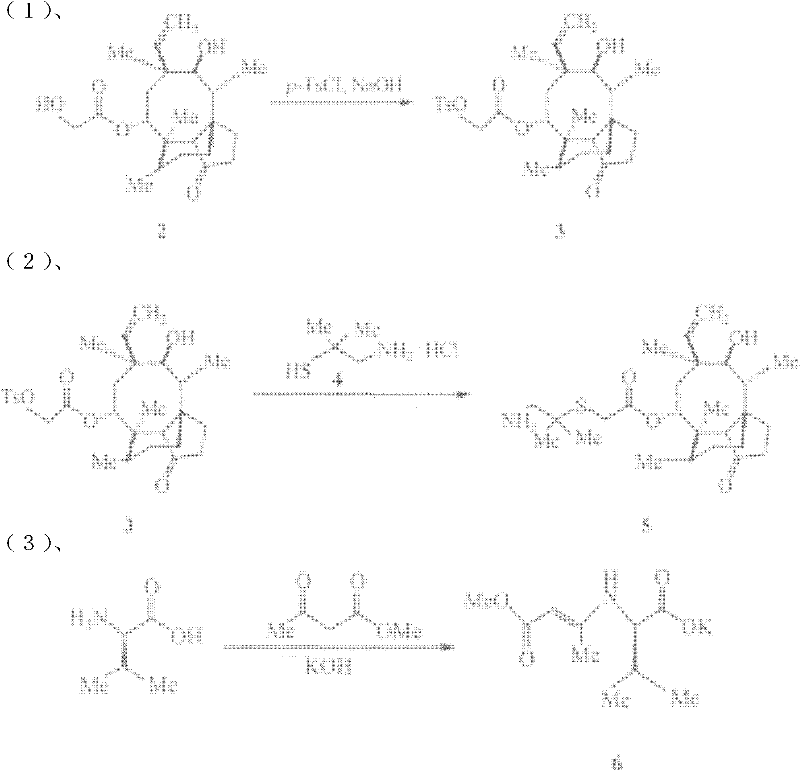
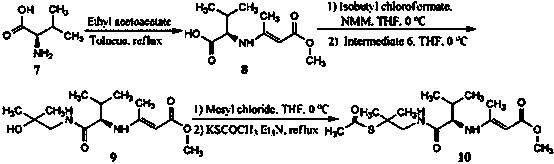
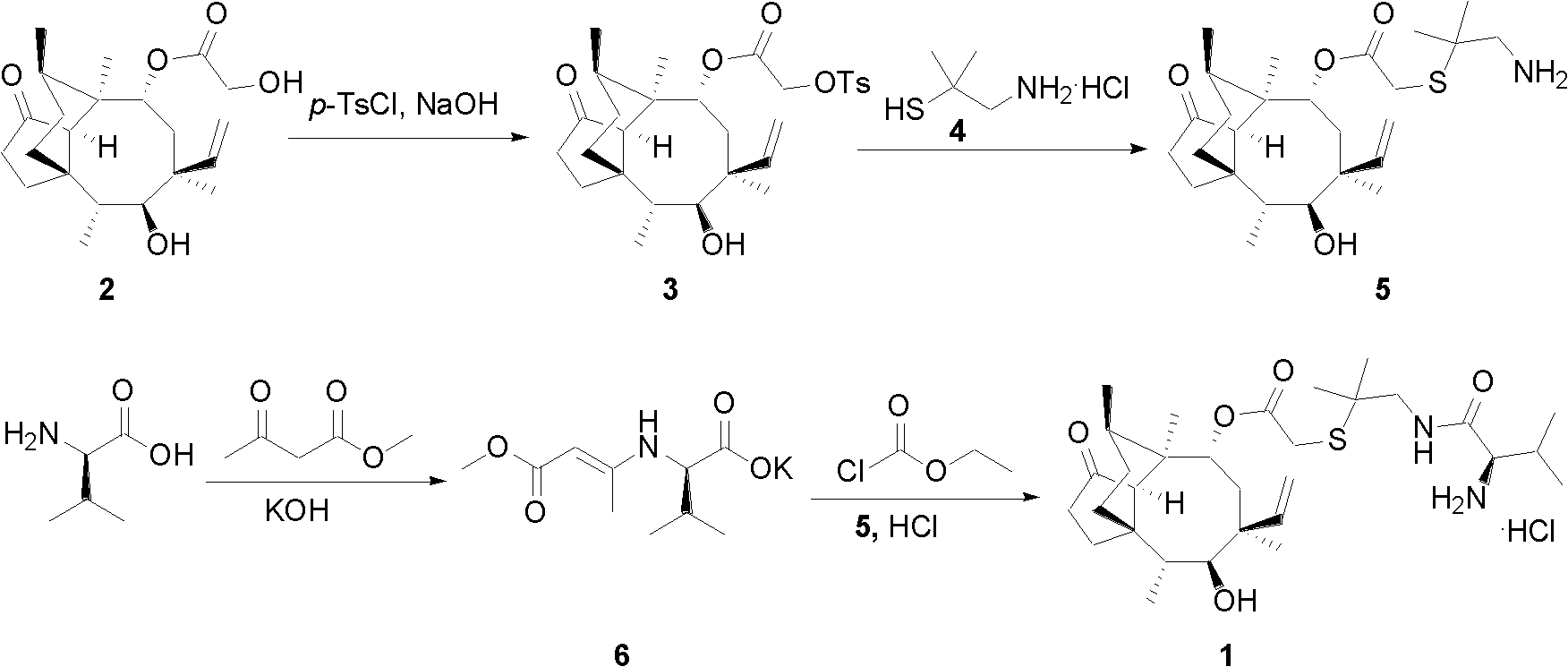
Chemical synthesis method of valnemulin hydrochloride
The invention discloses a chemical synthesis method of valnemulin hydrochloride, which comprises the following steps of: taking refined pleuromutilin as raw material, carrying out sulfonation by paratoluensulfonyl chloride, and reacting with dimethyl cysteamine hcl, to obtain the pleuromutilin dimethyl cysteamine substitute; reacting D-valine, methyl acetoacetate and potassium hydroxide to obtain (R)-2-(1-methoxycarbonyl group-2-allyl) amino-3-methyl potassium butyrate, activating by ethyl chloroformate and reacting with the pleuromutilin dimethyl cysteamine substitute, adjusting PH value, carrying out reverse phase extraction, and carrying out freeze-drying to obtain the valnemulin hydrochloride. The method has the advantages that due to the refining of the raw material pleuromutilin, the impurities in the product can be effectively removed, and the purifying process can be simplified from the source; the carboxyl of D-valine can be activated by the ethyl chloroformate, so that the reaction is easier to carry out; and due to the pH adjustment, the reverse phase extraction, and the freeze-drying, the product can be obtained, so that the product is stable in quality, and high in purity.
Owner:武汉回盛生物科技股份有限公司
Preparation method of valnemulin and hydrochloride thereof
The invention belongs to preparation of antibiotic, in particular to preparation method of Valnemulin and hydrochloride thereof.D-hydroxy valine Dane salt is added into more than ten weight of tetrahydrofuran and is suspended by stirring at 0-20 DEG C, and triethylamine, N, N-dimethyl formamide (acetamide) and N-methyl morpholine are added until reaction liquid is clear; methyl (ethyl) chloroformate molar weight of which is not more than that of D-hydroxy valine Dane salt is added; (2-amino-1, 1-dimethyl ethyl) mercaptoacetic acid, (3aS, 4R, 5S, 6S, 8R, 9R, 9aR, 10R)-octohydrogen-5, 8-dyhydroxy-4, 6, 9,10-tetramethyl-6-ethenyl-3a, 9-propane-3aH-cyclopentene (cyclooctene)-1(4H)-keto-8-ester the molar weight of which is not more than that of D-hydroxy valine Dane salt is dissolved in equal mass of tetrahydrofuran, the reaction liquid is dripped in and stirred at 0-10 DEG C, and reaction is carried out for 2-5h at 0-20 DEG C; the organic solvent is removed, the residue is added with water and hydrochloric acid is used for adjusting pH value, heating up, stirring and decontaminating are carried out, and the filtration is freeze dried to obtain Valnemulin hydrochloride. The invention solves the problems of low purity and high reaction condition of the existing product and has the advantages of simple technology and high product purity.
Owner:河北远征禾木药业有限公司
Preparation method of valnemulin hydrochloride
InactiveCN102225905AReduce pollutionMild reaction temperatureSulfide preparationReaction temperatureSolvent
The invention relates to a preparation method of valnemulin hydrochloride. The preparation method comprises the following steps: reacting pleuromutilin with p-toluenesulfonyl chloride, substituting with 1-amino-2-methyl propyl-2-mercaptan hydrochloride so as to obtain [(2-amino-1,1-dimethyl S, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester for later use; reacting D-valine with methyl acetoacetate; synthesizing the product with isobutyl chloroacetate so as to generate anhydride; and reacting anhydride with [(2-amino-1,1-dimethyl ethyl) sulfenyl] acetic acid (3aS, 4R, 5, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester so as to generate amide, and then carrying out deprotection with hydrochloric acid so as to prepare valnemulin hydrochloride. The method has the advantages that (1) reaction temperature is mild, thereby being applicable to large-scale production; (2) post-treatment is simple, thereby directly obtaining the product; (3) used solvents are less, thereby reducing environmental pollution; and (4) the valnemulin hydrochloride content and yield of the obtained product are high.
Owner:HUBEI SHENZHOU CHEM
Method for preparing valnemulin hydrochloride coating granule
InactiveCN101601658ATo achieve the purpose of taste maskingLong stable timeAntibacterial agentsPharmaceutical non-active ingredientsValnemulin HydrochlorideIrritation
The invention belongs to a process for preparing a valnemulin hydrochloride premix of pleuromutilin antibiotics specially used for animals, and in particular relates to a method for preparing a valnemulin hydrochloride coating granule. The method comprises the following process steps of pre-granulation of the valnemulin hydrochloride, preparation of coating solution, coating of the valnemulin hydrochloride and the like. The method has the advantages of solving the problems existing in the prior art that the valnemulin hydrochloride materials are hygroscopic, sensitive to light and strong in powder irritation, and are easily decomposed when being mixed with feed and the like, effectively achieving the aim of odor masking and the like; moreover, after coating, the coating granule is stable under conditions of a light accelerating experiment.
Owner:河北远征药业有限公司
Method for purifying valnemulin hydrochloride
ActiveCN102344397AEffective and stable purificationEasy to purifyOrganic chemistryOrganic compound preparationAlcoholPhosphate
The invention provides a method for purifying valnemulin hydrochloride. The method comprises the following steps of: directly alkalizing crude valnemulin in an aqueous solution thereof; and directly preparing into phosphate in an alcohol solution and curing. In the method, multiple times of extraction of valnemulin hydrochloride and introduction of other salts are not required, the treating process is easy and convenient, the loss of the product is small, waste water is easy to treat, and the problem of difficulty in purifying the valnemulin hydrochloride is solved.
Owner:浙江拜克生物科技有限公司
Veterinary-use intestinal targeted antibacterial pellet and preparation method thereof
ActiveCN102600184AReduce first pass effectImprove bioavailabilityAntibacterial agentsTetracycline active ingredientsFormularyChlortetracycline Hydrochloride
The invention discloses a veterinary-use intestinal targeted antibacterial pellet. The veterinary-use intestinal targeted antibacterial pellet comprises a main drug and auxiliary drugs, wherein the main drug is composed of valnemulin hydrochloride and chlortetracycline hydrochloride; the mass of the valnemulin hydrochloride is 1-5% of that of the pellet; the mass of the chlortetracycline hydrochloride is 5-16 times of that of the valnemulin hydrochloride; the pellet sequentially comprises a valnemulin hydrochloride core, an intestinal targeted coating layer, a chlortetracycline hydrochloride drug layer and an outer coating layer from inside to outside. The invention further discloses a preparation method of the veterinary-use intestinal targeted antibacterial pellet. Through the combination of the method and the formula, the valnemulin hydrochloride and the chlortetracycline hydrochloride are adopted for preparing the pellet; the pellet is released in a gradient form in an animal digestion system; the chlortetracycline hydrochloride is firstly released and absorbed by the stomach and the intestine; the valnemulin hydrochloride is rapidly released and absorbed by the intestinal tract with the pH value larger than or equal to 6.8, so that an excellent intestinal tract sterilization effect is achieved, the drug absorbed by the colon can be prevented from suffering from the first-pass effect of the liver and the intestine, the haemoconcentration and bioavailability of the valnemulin hydrochloride can be improved, and an excellent treatment effect for systemic diseases is obtained.
Owner:山东胜利生物工程有限公司
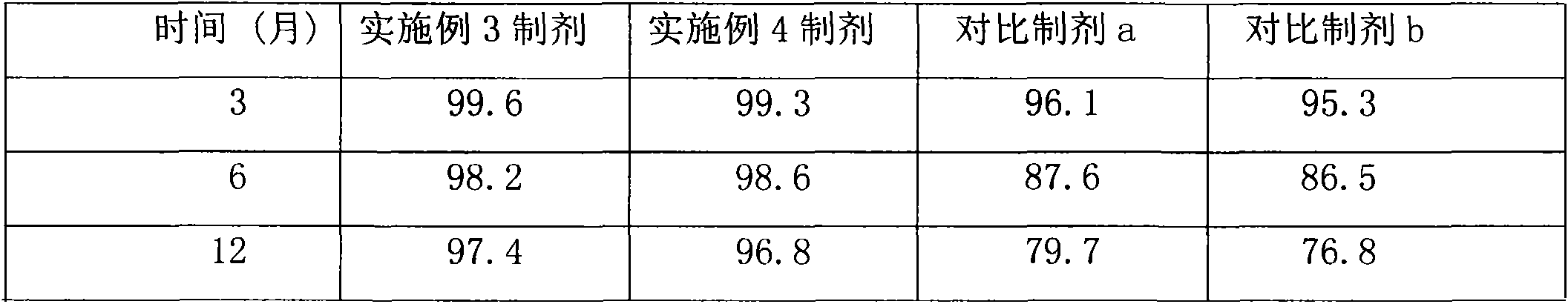
Crystalline warnemulin hydrochloride product and crystallization preparation method thereof
ActiveCN104876841BHigh bulk densityImprove stabilityOrganic chemistryOrganic compound preparationX-rayRaw material
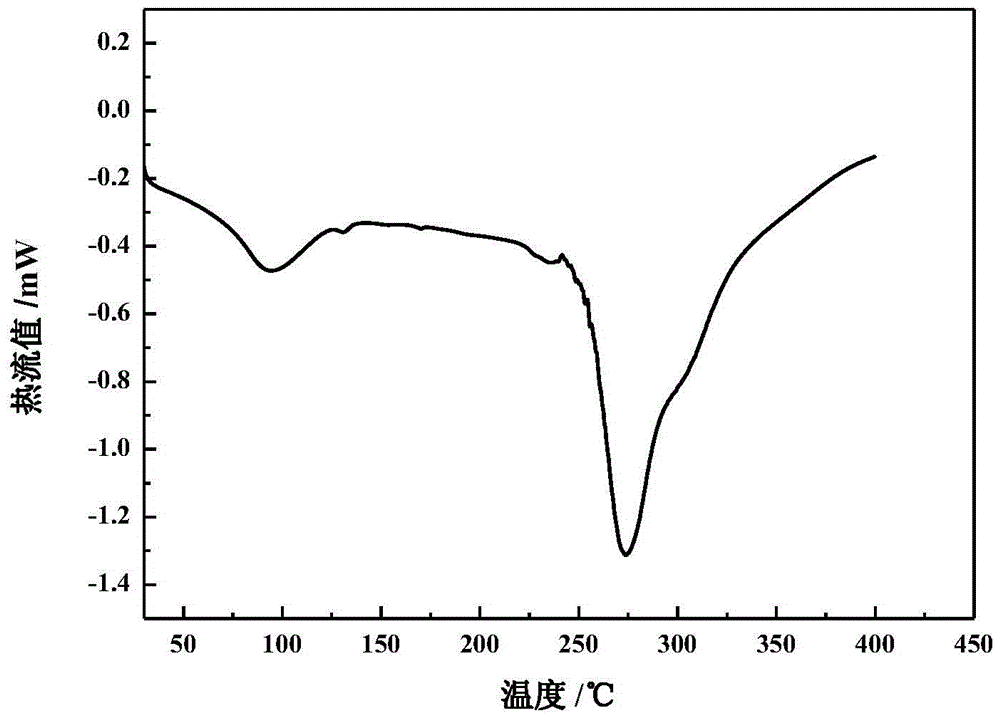
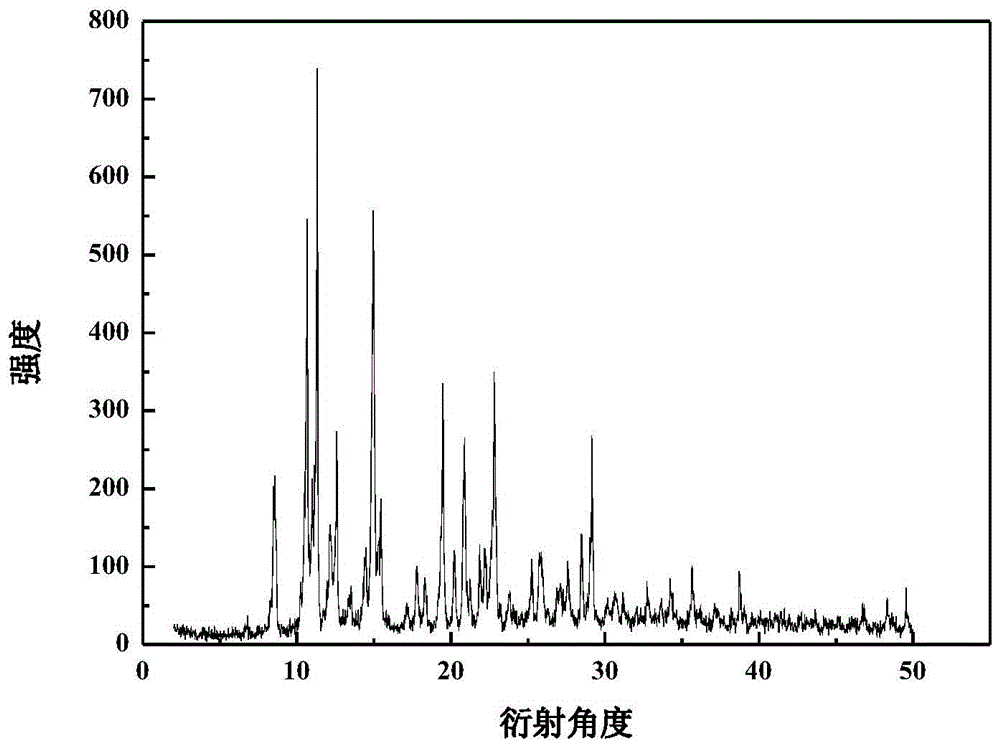
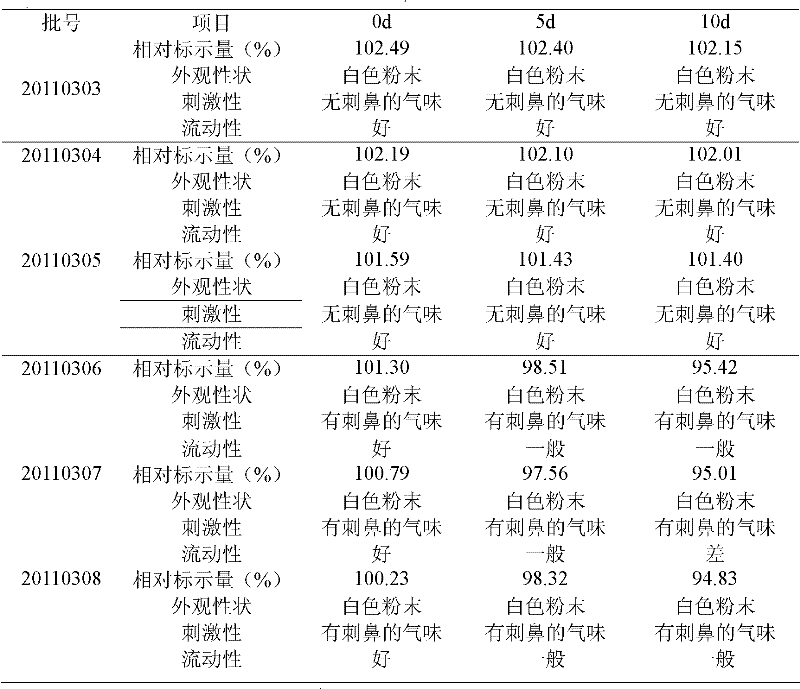
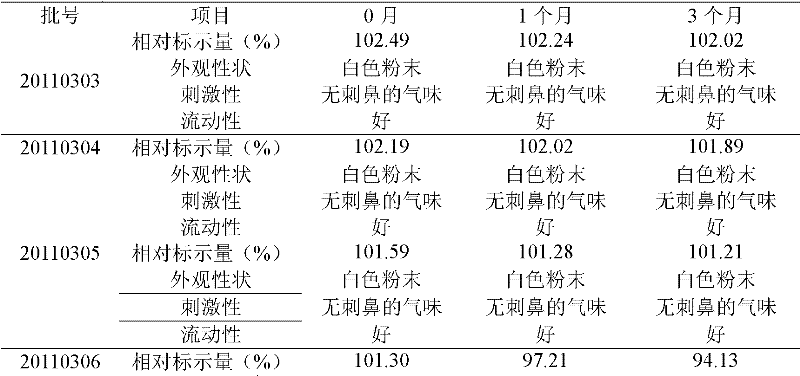
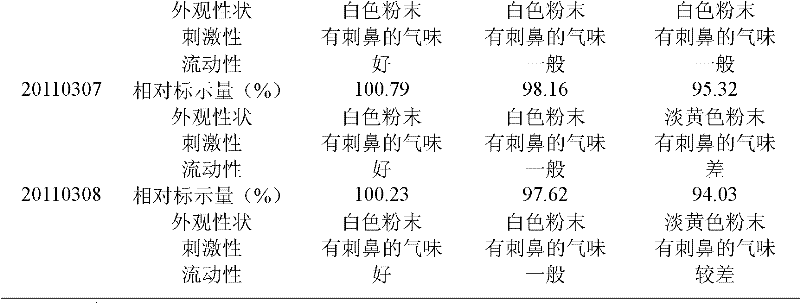
The invention relates to a crystalline valnemulin hydrochloride product and a crystallization preparation method thereof. The crystalline valnemulin hydrochloride product is defined by characteristic peaks of an X-ray powder diffraction spectrum in 2theta and DSC. The crystallization preparation method comprises the following steps: under the stirring action, at the temperature of 40-60 DEG C, dissolving an amorphous valnemulin hydrochloride raw material in some organic solvent, and stirring for 1 hour to be completely dissolved, wherein the final solution concentration is 0.5-1 g / mL; at the temperature of 40-60 DEG C, stirring and adding an ultrasonic unit into the solution to be subjected to ultrasonic treatment for 1-2 hours; then reducing the solution temperature to the final temperature of 5-15 DEG C at some cooling rate; continuously stirring at the final temperature, growing the grain for 1-2 hours, and washing, filtering and drying crystal mush to obtain the crystalline valnemulin hydrochloride product. The crystalline valnemulin hydrochloride product is relatively white in color, uniform in granularity, good in flowability and stability, low in hygroscopicity, and high in bulk density, and the once through yield during the crystallization process is higher than 90%.
Owner:TIANJIN UNIV +1
Synthesis method for valnemulin hydrochloride
InactiveCN103382172ALow toxicityNo pollution in the processSulfide preparationCysteamineValnemulin Hydrochloride
The invention discloses a synthesis method for valnemulin hydrochloride. The synthesis method comprises the steps: with pleuromutilin, dimethyl cysteamine and D-valine as main raw materials, carrying out amino protection, pleuromutilin activation, hydrocarbylation, acylation and deamination protection reactions, and ultimately preparing to obtain valnemulin hydrochloride. The synthesis method is suitable for industrialized production, and has the main advantages that: 1, during the reaction process and without need of column separation, valnemulin hydrochloride with higher content is obtained; 2, a used solvent has small toxicity, can also be recycled, does not pollute the environment, and is suitable for industrialized production; 3, a reaction condition is mild and is generally between 0-70 DEG C, and higher temperature and lower temperature reactions does not exist; and 4, the total yield is higher and reaches 50% or more.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
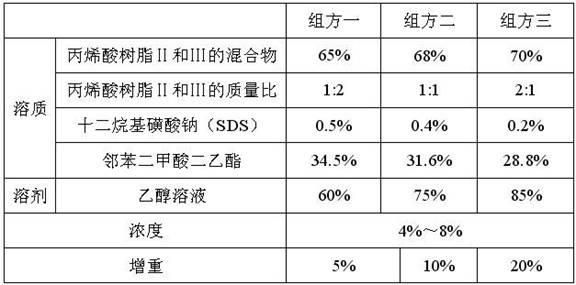
Recipe of valnemulin hydrochloride enteric-coated pellet and preparation method thereof
InactiveCN101874785AMask bitter off-flavorsImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsHigh concentrationPrill
The invention provides a recipe of a valnemulin hydrochloride enteric-coated pellet and a preparation method thereof, wherein, acrylic resin water dispersion is taken as coating liquid, and water is taken as a solvent, thus changing the method that the existing resin coating liquid selects high concentration alcohol as the solvent, lowering cost and overcoming the defect of environmental influence due to evaporation of a large amount of alcohol during the coating process. The preparation method of the valnemulin hydrochloride enteric-coated pellet comprises the steps of preparation of the valnemulin hydrochloride medicine carrying pellet, preparation of the coating liquid and preparation of valnemulin hydrochloride coating. By adopting an extrusion-spheronization process procedure, the preparation method solves the defects of a one-step fluidized bed granulating method such as coarse and loose particles, large particle size range, a large amount of dust; and the pellet prepared by the method has even particle size and narrower distribution range, thus being beneficial to uniform saturation of the coating liquid on the particles, and improving the taste masking effect.
Owner:广东大华农动物食品保健品股份有限公司 +1
Valnemulin hydrochloride enteric solid dispersion, as well as preparation method and application thereof
ActiveCN103751092AMild conditionsLow priceAntibacterial agentsPharmaceutical delivery mechanismLiquid productOrganic solvent
The invention belongs to the technical field of special antibacterial preparations for animals, and discloses a valnemulin hydrochloride enteric solid dispersion, as well as a preparation method and application thereof. The preparation method comprises the concrete steps of dissolving valnemulin hydrochloride and an enteric carrier in an organic solvent, and preparing a mixed liquid product by a solvent method; removing the organic solvent by using a rotary evaporator; grinding and screening to obtain the valnemulin hydrochloride enteric solid dispersion. The valnemulin hydrochloride enteric solid dispersion prepared by the method can be used for preparing special antibacterial premixes for animals. The preparation method is low in raw material cost and simple in production equipment and process operation; the prepared valnemulin hydrochloride enteric solid dispersion is stable in property, good in uniformity and large in mobility, does not have pungent smell or hygroscopicity, and is hardly released in an acid medium and almost completely released in a buffer salt medium.
Owner:SOUTH CHINA AGRI UNIV
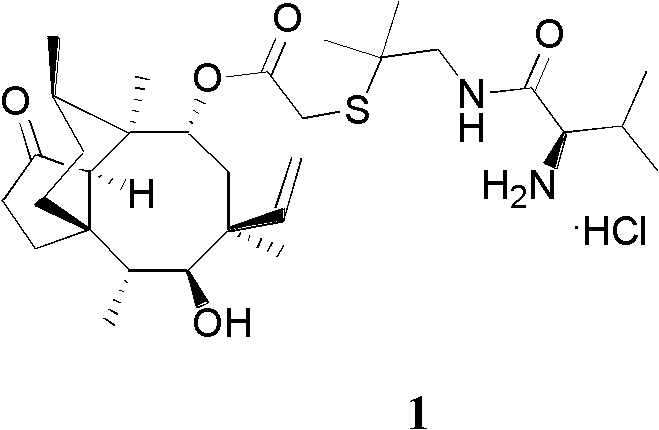
Application of valnemulin hydrochloride
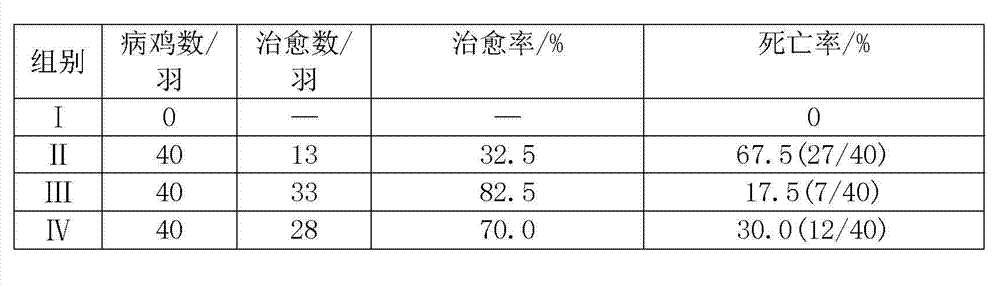
InactiveCN102764252AResidue reductionNo drug resistanceAntibacterial agentsEster active ingredientsDiseaseTreatment effect
The invention provides an application of valnemulin hydrochloride which is utilized to prepare a medicine for treating and preventing chicken mycoplasma infection and bacterial infection diseases. The medicine prepared by the valnemulin hydrochloride is a premix, soluble powder and a solution. Compared with traditional technology, the valnemulin hydrochloride provided by the invention is applied to chicken respiratory disease and chicken bacterial infection disease, so that the treatment effect of the valnemulin hydrochloride is more significant and the two diseases can be completely healed under the reasonable dose; and the residual quantity of the medicine in the chicken bodies is very low after the chicken is healed, the healed chicken is free from drug resistance of the medicine, so that the medicine can be repeatedly utilized but the curative effect of the medicine is not influenced.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Refining method of valnemulin hydrochloride
ActiveCN103483232ASignificant purificationGood purification effectOrganic compound preparationSulfide preparationInorganic saltsValnemulin Hydrochloride
The invention relates to a refining method of valnemulin hydrochloride. The refining method comprises the following steps: adding purified water or distilled water into an ether solution of valnemulin alkali; mixing; heating the solution until the temperature of the solution reaches 40-50 DEG C; stirring for 0.5-1h; standing for layering; taking the supernatant; adding an ethanol solution into the supernatant; stirring for 0.5-1h; standing for layering; taking the supernatant organic liquid; leading dry hydrogen chloride gas into the supernatant organic liquid; slowly stirring for 2-3h at the room temperature; separating out a large number of crystals; slowly reducing the temperature of the solution to 0 DEG C; filtering after completely crystallizing; drying the crystals. According to the refining method, the characteristics that the valnemulin alkali can be dissolved in the ether solution and the valnemulin hydrochloride cannot be dissolved in the ether solution are utilized, the valnemulin is become into a salt and then separated out through crystallization; the water and the ethanol solution are used for washing the valnemulin alkali so as to remove inorganic salt impurities and organic impurities from the valnemulin alkali, thus achieving a purifying purpose. The refining method has the characteristics of simple process, easy implementation in production, low cost, no pollution, relatively-high yield and high product purity.
Owner:宁夏泰瑞制药股份有限公司
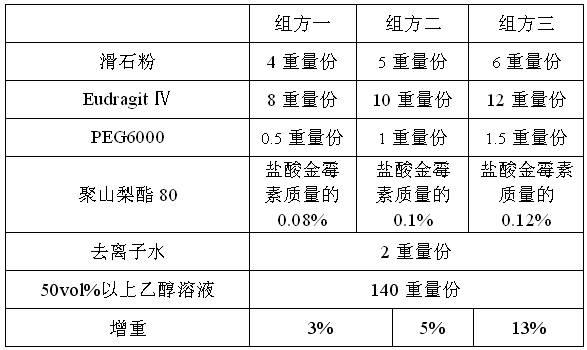
A kind of warnemulin hydrochloride premix for animals and preparation method thereof
ActiveCN102266319AImprove adhesionHigh encapsulation efficiencyAntibacterial agentsEster active ingredientsPolyethylene glycolSilica gel
The invention relates to the technical field of veterinary antibiotic preparations, and particularly discloses a veterinary valnemulin hydrochloride premix and a preparation method thereof. The premix comprises a valnemulin hydrochloride clathrate and a matrix diluent, wherein the coating carrier of the clathrate is any one of or a mixture of two of beta-cyclodextrin, hydroxypropyl beta-cyclodextrin, polyethylene glycol 4000, polyethylene glycol 6000, and ethyl cellulose; and the matrix diluent is any one of or a mixture of two or three of starch, maltodextrin, miropowder silica gel and talcum powder. Anhydrous ethanol and liquid state polyethylene glycol are added in the clathrate to promote the bonding of the clathrate, and wetting agent sorbitol is added to form a semi-solid state, so as to increase the molecular acting force between the entire medicine and a polymer carrier and increase the encapsulation rate of the clathrate. The prepared veterinary valnemulin hydrochloride premix has the advantages of stable properties, good uniformity, high fluidity, and no pungent odor.
Owner:武汉回盛生物科技股份有限公司
Carnauba wax-containing valnemulin injection
ActiveCN104906590AAntibacterial agentsPharmaceutical delivery mechanismGlyceryl monostearateSodium citrate dihydrate
The invention relates to a carnauba wax-containing valnemulin injection with latex-like appearance. Each liter of the injection comprises 150-180g of valnemulin hydrochloride, 2.5-11g of carnauba wax, and the balance of glyceryl triacetate / benzyl benzoate / 1,2-propylene glycol / ethanol with a volume ratio of 4.5:3:3.3:1. Each of the injection also comprises 100-150ml of glyceryl monooleate. In the preparation process of 1L of the injection, 13.0-31.2g of sodium citrate dihydrate (C6H5Na3O7.2H2O) or 19.0-31.9g of sodium carbonate decahydrate (Na2CO3.10H2O) is added. Added sodium citrate or sodium carbonate reacts with valnemulin hydrochloride. Each liter of the injection prepared in the invention includes 150-180g of valnemulin alkali and valnemulin acid salt (hydrochloride or citrate), 2.5-11g of carnauba wax, 100-150ml of glyceryl monooleate, and the balance of glyceryl triacetate / benzyl benzoate / 1,2-propylene glycol / ethanol with a volume ratio of 4.5:3:3.3:1.
Owner:北京中农华威科技集团有限公司
Valnemulin hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN108635333AGood resolubilityQuality improvementAntibacterial agentsPowder deliverySolubilityValnemulin Hydrochloride
The invention discloses a valnemulin hydrochloride freeze-dried powder injection and a preparation method thereof. The preparation method comprises the following steps: dissolving the proportioned valnemulin raw powder and excipient by adopting injection water, removing the heat source, obtaining the valnemulin hydrochloride freeze-dried stock solution, pre-freezing, subliming drying, parsing drying, obtaining the freeze-dried powder, plugging, rolling a cover, inspecting, and obtaining a valnemulin hydrochloride freeze-dried powder injection finished product. The finished product appearance is in a white loose block shape, can be rapidly dissolved into clear liquid after the injection water is added, and is good in re-solubility. When the valnemulin hydrochloride freeze-dried powder injection is stored for six months under the condition of 40 plus or minus 2 DEG C and RH75 percent plus or minus 5 percent, the quality is stable. The valnemulin hydrochloride freeze-dried powder injection is simple, easy, stable and reliable in process, accurate in administration dosage of a dosage form, and suitable for the industrialized production.
Owner:四川通达动物保健科技有限公司
Uses of valnemulin hydrogen tartrate in veterinary drugs
InactiveCN102813644AWeak moistureResidue reductionAntibacterial agentsEster active ingredientsDiseaseHydrogen
The present invention provides uses of valnemulin hydrogen tartrate in veterinary drugs, and mainly relates to uses of the valnemulin hydrogen tartrate in preparations of drugs for treatments and preventions of veterinary diseases related to swine mycoplasma infection and bacterial infection. Compared to the prior art, the following characteristics in the present invention are provided: valnemulin hydrogen tartrate is made into a premix or a granule or powder, a mixing feeding way is adopted to achieve prevention and treatment of swine mycoplasma infection and bacterial infection, an anti-mycoplasma effect of the drug of the present invention is better than anti-mycoplasma effects of other similar products, and no drug resistance is generated. According to the present invention, the uses of the valnemulin hydrogen tartrate in veterinary diseases in the present invention are not presented in the prior art; compared with valnemulin hydrochloride, preparations prepared by the valnemulin hydrogen tartrate provide better effects for treatments of veterinary diseases, and especially for swine diseases; hygroscopicity of the valnemulin hydrogen tartrate is low, and is easily stored for a long time, wherein effects of the drug can not be affected; and palatability of the preparations prepared by the valnemulin hydrogen tartrate is stronger than palatability of valnemulin hydrochloride preparations in the prior art.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Synthesis of intermediate compound of warnemulin hydrochloride and preparation method of warnemulin hydrochloride
InactiveCN106496085BOrganic compound preparationSulfonic acid esters preparationChemical structurePropylamine
The invention discloses an intermediate compound used for synthesizing valnemulin hydrochloride. The chemical structure of the intermediate compound used for synthesizing valnemulin hydrochloride is represented by a formula in the invention. A preparation method of the intermediate compound comprises following steps: beta-hydroxyisovaleric acid is subjected to Curtius rearrangement reaction so as to obtain 2-methyl-2-hydroxy propylamine; 2-methyl-2-hydroxy propylamine and D-valine Dane salt are subjected to mixed anhydride method reaction so as to obtain an amide product; the amide product is subjected to hydroxy activation under alkaline conditions with methylsulfonyl chloride, and then is reacted with potassium thioacetate. The preparation method of valnemulin hydrochloride comprises following steps: pleuromutilin is reacted with paratoluensulfonyl chloride so as to obtain pleuromutilin p-tosylate; pleuromutilin p-tosylate is reacted with the intermediate compound, and hydrochloric acid deprotection is carried out so as to obtain valnemulin hydrochloride. In the preparation method of valnemulin hydrochloride, no dimethyl cysteamine hydrochloride is needed, production conditions are mild, no environment pollution is caused, and the preparation method is suitable for industrialized production.
Owner:盐城市优化医药化工科技有限公司
Valnemulin hydrochloride granule and preparation method thereof
InactiveCN102406618AAvoid odorOvercome the smellAntibacterial agentsGranular deliveryFiller ExcipientValnemulin Hydrochloride
The invention discloses a valnemulin hydrochloride granule which comprises the following raw materials by weight: 10% of valnemulin hydrochloride, 89% of fillers, and 1% of wetting agents. The invention also discloses a preparation method of the valnemulin hydrochloride granule. Since valnemulin hydrochloride itself has heavy peculiar smell and is unstable, the invention prepares granules to overcome the peculiar smell of valnemulin hydrochloride, and improves the stability; no dosage form administrated in a granule form is on the market; the invention introduces a dosage form of a same medicament, makes the administration become more diverse, and meets the deficiency in the market. The valnemulin hydrochloride granule of the invention has the advantages of fast absorption, quick effect, capability of overcoming the deficiencies of former dosage forms, convenience, stability, and good taste.
Owner:上海恒丰强生物技术有限公司
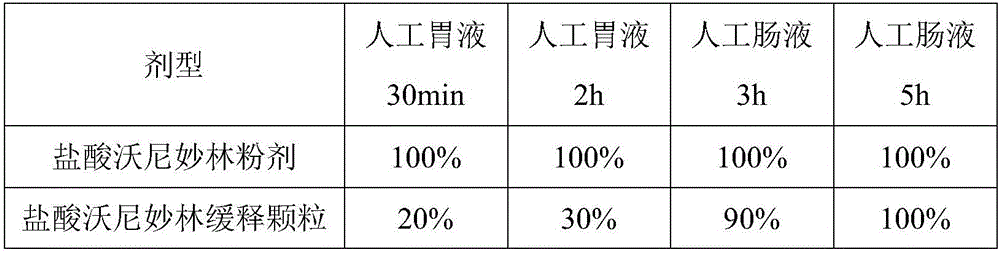
Valnemulin hydrochloride sustained-release granules and preparation method and application thereof
ActiveCN105796504AImprove stabilityEasy to storeAntibacterial agentsGranular deliveryVegetable oilMonoglyceride
The invention discloses valnemulin hydrochloride sustained-release granules and a preparation method and application thereof.The valnemulin hydrochloride sustained-release granules are prepared from, by mass, 5-50% of valnemulin hydrochloride and the balance carrier auxiliary materials through the fluidized spraying technology.The carrier auxiliary materials are one or more of animal fat, beewax, carnauba wax, hydrogenated vegetable oil, stearyl alcohol, fatty acid monoglyceride, fatty acid di-glyceride and paraffin.The valnemulin hydrochloride sustained-release granules solve the problem that medicine stability is poor, improve product quality and stability, and prolong service life.The problems that medicine powder is high in irritation and poor in palatability are solved.The granules and feed are mixed to be supplied, the medicine is released slowly, effective blood concentration maintenance time is longer, the administration frequency is reduced, the bioavailability of valnemulin hydrochloride is improved, and the clinical use effect is remarkable.
Owner:JINHE ANIMAL PHARMA
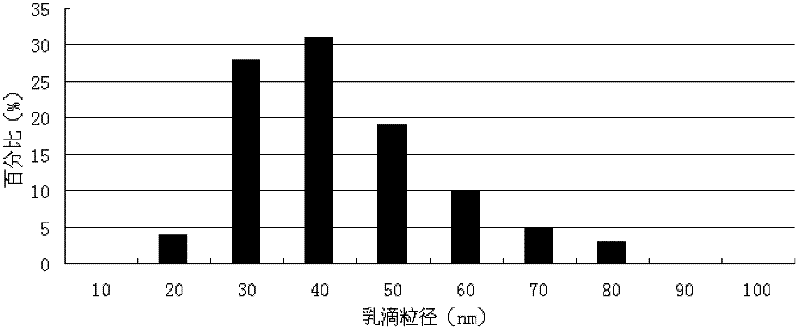
Valnemulin hydrochloride self-emulsified oral nano emulsion for veterinary use and preparation method thereof
ActiveCN102415996AImprove stabilityAvoid hydrolysisAntibacterial agentsEmulsion deliveryDecompositionValnemulin Hydrochloride
The invention discloses valnemulin hydrochloride self-emulsified oral nano emulsion for veterinary use and a preparation method thereof. The valnemulin hydrochloride self-emulsified oral nano emulsion for veterinary use comprises the following components: valnemulin hydrochloride, oil, surfactant, preservative, antioxygen and auxiliary surfactant. After disintegrating in water and being stirred slightly or being taken orally and moving with intestine movement, the self-emulsified emulsion can spontaneously form emulsion with emulsion droplet size of 10 to 100 nanometers; the drawbacks of decomposition of valnemulin during long standing and content reduction of the valnemulin or valnemulin hydrochloride nano emulsion prepared in the prior art are overcome and the content stability of the product is increased; the palatability of the valnemulin product is improved; and the preparation process is simple, and the valnemulin hydrochloride self-emulsified oral nano emulsion is suitable to be produced in large scale by veterinary medicine enterprises.
Owner:QILU ANIMAL HEALTH PROD
A kind of synthetic method of warnemulin hydrochloride
ActiveCN106432017BHigh yieldHigh purityOrganic compound preparationSulfonic acid esters preparationSynthesis methodsHydrolysis
The invention discloses a synthesis method of valnemulin hydrochloride. The synthesis method comprises the following three steps: synthesis of dimethylcysteamine pleuromulin from pleuromulin, synthesis of D-valine dane salt from D-valine and synthesis of valnemulin hydrochloride from dimethylcysteamine pleuromulin and D-valine dane salt. The synthesis method has the advantages that lots of homogeneous reactions are adopted, the post-treatment method is simple, organic solvents can be recycled, the reaction yield and the product purity are high, safety and environment-friendliness are realized, the reaction is moderate, and thus, the method is very suitable for industrialized enlarged production; with the adoption of the method, the repeated washing step during D-valine hydrolysis and salt formation is omitted, the production operation is simplified, the labor intensity is reduced, the consumption of low-boiling-point ether solvents is greatly reduced, volatilization of the solvents is reduced, low pollution is caused, and the method is conducive to the health of workers and the safety of the environment.
Owner:河北远征禾木药业有限公司
Chemical synthesis method of valnemulin hydrochloride
ActiveCN102675173BEfficient removalEasy to purifySulfide preparationChemical synthesisEthyl chloroformate
The invention discloses a chemical synthesis method of valnemulin hydrochloride, which comprises the following steps of: taking refined pleuromutilin as raw material, carrying out sulfonation by paratoluensulfonyl chloride, and reacting with dimethyl cysteamine hcl, to obtain the pleuromutilin dimethyl cysteamine substitute; reacting D-valine, methyl acetoacetate and potassium hydroxide to obtain (R)-2-(1-methoxycarbonyl group-2-allyl) amino-3-methyl potassium butyrate, activating by ethyl chloroformate and reacting with the pleuromutilin dimethyl cysteamine substitute, adjusting PH value, carrying out reverse phase extraction, and carrying out freeze-drying to obtain the valnemulin hydrochloride. The method has the advantages that due to the refining of the raw material pleuromutilin, the impurities in the product can be effectively removed, and the purifying process can be simplified from the source; the carboxyl of D-valine can be activated by the ethyl chloroformate, so that the reaction is easier to carry out; and due to the pH adjustment, the reverse phase extraction, and the freeze-drying, the product can be obtained, so that the product is stable in quality, and high in purity.
Owner:武汉回盛生物科技股份有限公司
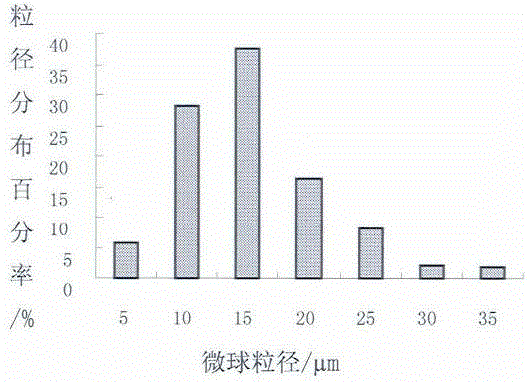
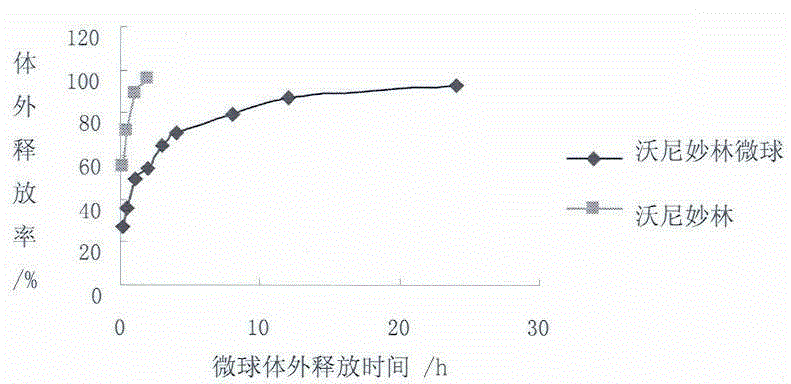
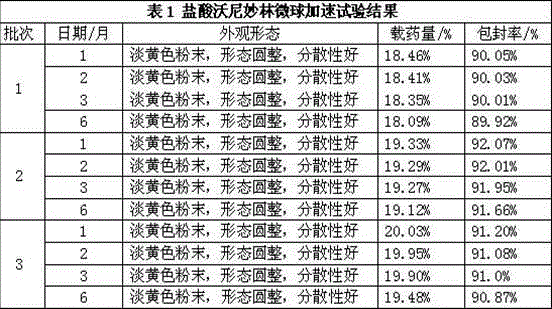
Valnemulin hydrochloride microspheres and preparation method thereof
ActiveCN104055737ALess irritatingReduce releaseAntibacterial agentsPharmaceutical non-active ingredientsMicrospherePyrrolidinones
The invention relates to valnemulin hydrochloride microspheres and preparation method thereof. The valnemulin hydrochloride microspheres are composed of a water phase, an oil phase and a crosslinking agent, wherein a volume ration of the water phase to the oil phase is 1:6-12. The water phase is composed of gelatin, valnemulin hydrochloride, polyvinylpyrrolidone and purified water and the oil phase is composed of an emulsifier and liquid paraffin. The preparation method includes following steps: preparing the water phase and the oil phase, adding the water phase dropwisely to the oil phase, adding the crosslinking agent, washing off the liquid paraffin on surfaces of the microspheres through petroleum ether and performing a vacuum-drying process to obtain the valnemulin hydrochloride microspheres.
Owner:LINYI UNIVERSITY
Valnemulin-O-benzoyl sulfonimide compound and preparation method thereof
InactiveCN107805226AImprove bioavailabilityReduce powder pungent odorOrganic chemistryClinical efficacyOrganic solvent
The invention provides a valnemulin-O-benzoyl sulfonimide compound. The compound is prepared by adopting a method comprising the following steps: firstly dissolving O-benzoyl sulfonamide in water, regulating a pH value to 4-5, adding an organic solvent for extraction, collecting an organic layer, then adding valnemulin into the organic layer for reaction, reducing the temperature to 0-5 DEG C after the end of reaction, performing suction filtration and collection on a solid precipitate and then drying to obtain the valnemulin-O-benzoyl sulfonimide compound. The valnemulin-O-benzoyl sulfonimidecompound provided by the invention solves the problem in the prior art, can improve the palatability of existing valnemulin hydrochloride, improve the bioavailability of the valnemulin and achieve the purpose of ensuring a clinical treatment effect.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
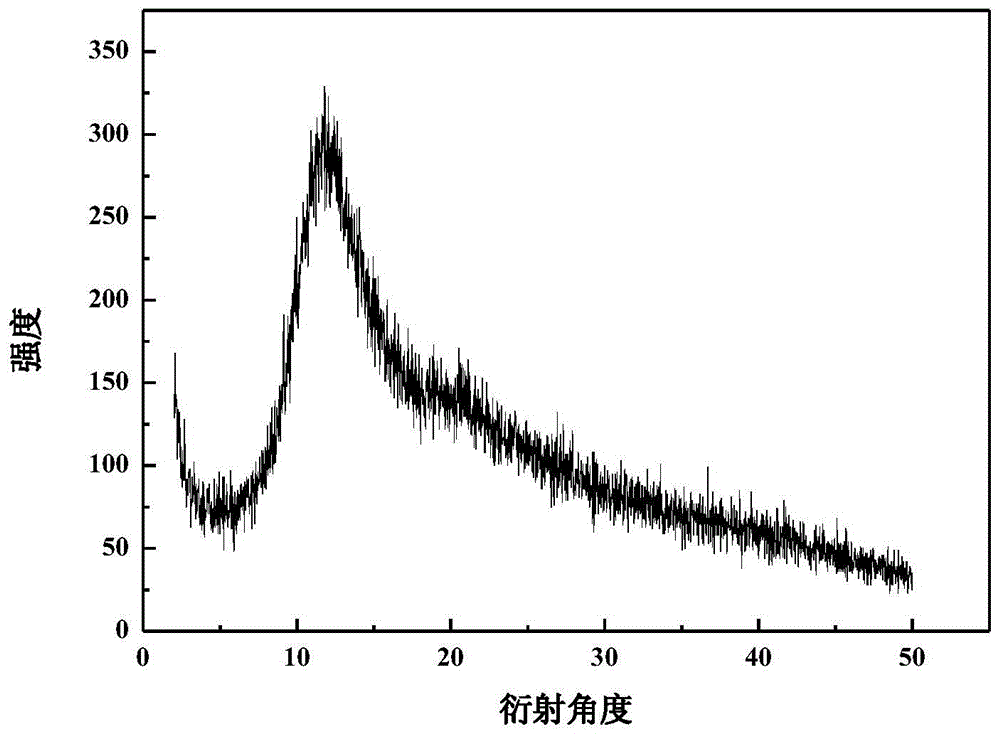
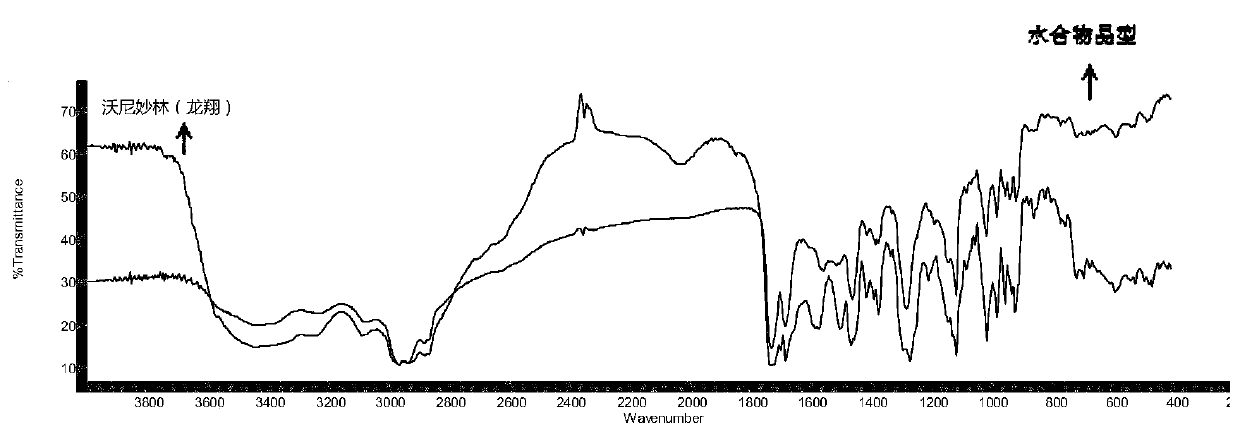
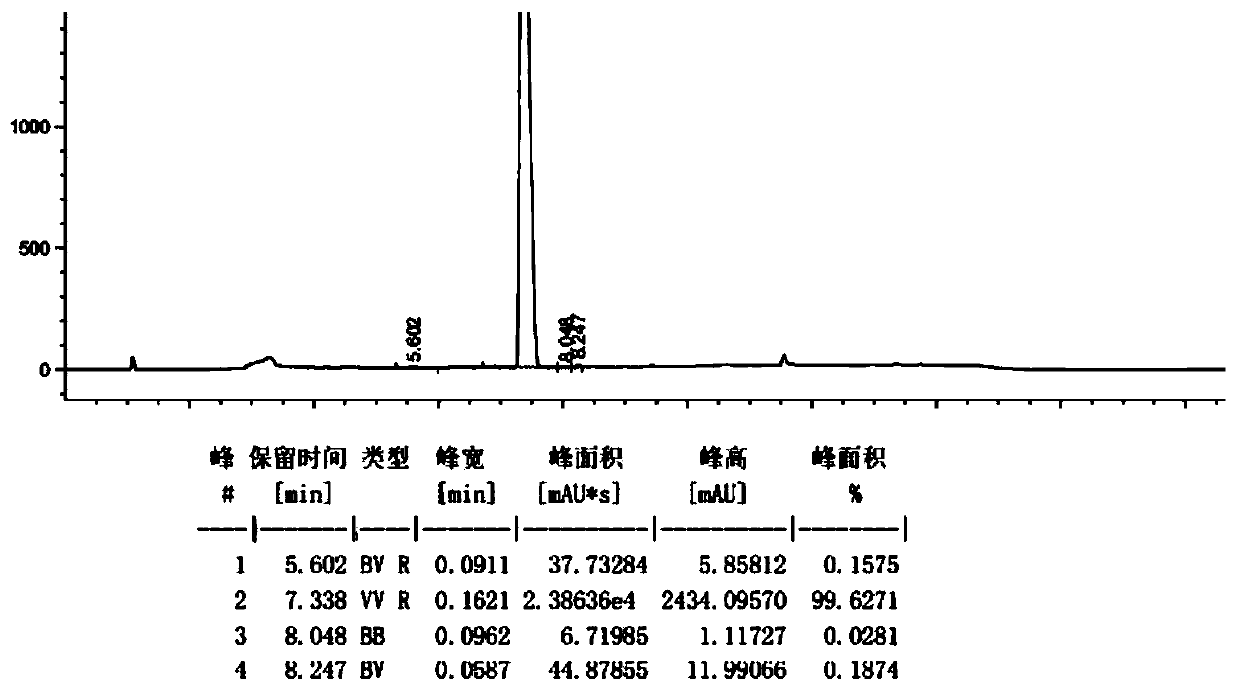
Valnemulin hydrochloride hydrate crystal form and preparation method thereof and pharmaceutical composition containing crystal form
ActiveCN110294697AEasy to operateEasy to makeAntibacterial agentsOrganic compound preparationValnemulin HydrochlorideX-ray
The invention relates to a valnemulin hydrochloride hydrate crystal form and a preparation method thereof and a pharmaceutical composition containing the crystal form. Specifically, the valnemulin hydrochloride hydrate crystal form shows characteristic peaks 2 Theta at 9.2+ / -0.2 degrees, 9.5+ / -0.2 degrees, 10.3+ / -0.2 degrees, 11.8+ / -0.2 degrees, 12.3+ / -0.2 degrees, 12.8+ / -0.2 degrees, 15.1+ / -0.2 degrees, 17.5+ / -0.2 degrees and 18.2+ / -0.2 degrees in an X-ray powder diffraction pattern. The valnemulin hydrochloride hydrate crystal form has high bulk density, no hygroscopicity and good stability.The invention also provides the preparation method of the valnemulin hydrochloride hydrate crystal form and the pharmaceutical composition containing the crystal form. The composition can be used forpreparing various preparations, such as dosage forms of soluble powder, oral liquids, sustained-release particles, injections and the like, and has wide clinical application prospect.
Owner:TIANJIN RINGPU BIO TECH +1
Soluble powder of crystalline valnemulin hydrochloride and application thereof
The invention discloses soluble powder of crystalline valnemulin hydrochloride and application thereof. The soluble powder consists of valnemulin hydrochloride hydrate crystal, a flavoring agent and auxiliary materials. The soluble powder made of the crystalline valnemulin hydrochloride does not induce dampness, has good thermal stability and low production cost, has no pungent smell, does not need to be coated, has better water solubility, and is beneficial to take by drinking water for livestock and poultry groups. Meanwhile, the soluble powder medicine has good palatability, improves the compliance of livestock and poultry medicines, increases the water drinking amount and the feed intake of animals, ensures the medicine dosage of livestock and poultry, and further improves the curativeeffect of valnemulin hydrochloride in prevention and treatment of mycoplasma infection and sensitive bacterial infection diseases of livestock and poultry.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Valnemulin hydrochloride solution and preparation method of same
InactiveCN102415989AIncrease fat solubilityImprove bioavailabilityAntibacterial agentsPharmaceutical delivery mechanismOrganic solventValnemulin Hydrochloride
The invention discloses a valnemulin hydrochloride solution. The valnemulin hydrochloride solution consists of the following raw materials in percentage by weight: 5% of valnemulin hydrochloride and 95% of polyglycol. The invention also discloses a preparation method of the valnemulin hydrochloride solution. In the invention, an organic solvent is adopted to dissolve the valnemulin hydrochloride, so that the valnemulin hydrochloride exists in a manner of solution, and is administered in the form of oral liquid. The valnemulin hydrochloride has the characteristics of high liposolubility, difficulty in dissolving in water and general organic solvents, low premix dissolution rate and low bioavailability, but solvent of the valnemulin hydrochloride developed by a pharmaceutical method is convenient to administer, and has high bioavailability.
Owner:上海恒丰强生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com