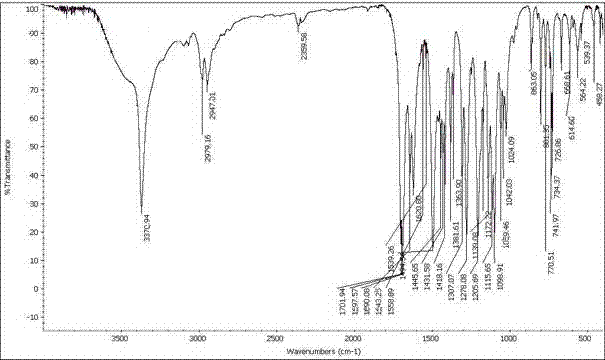
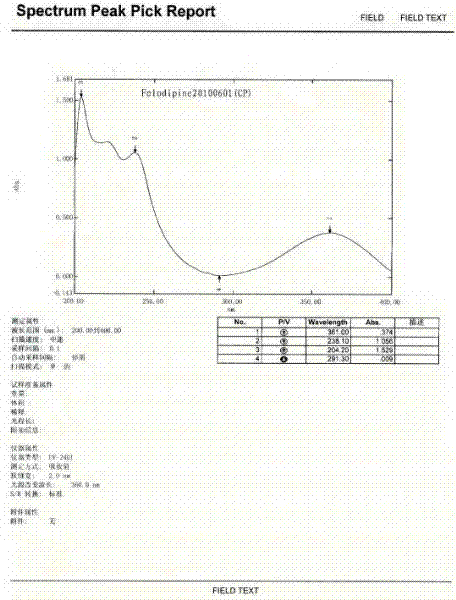
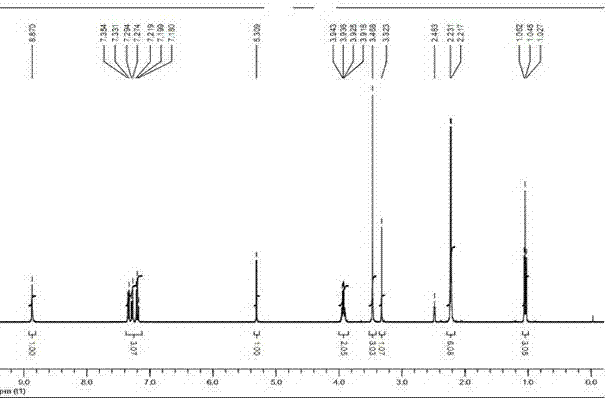
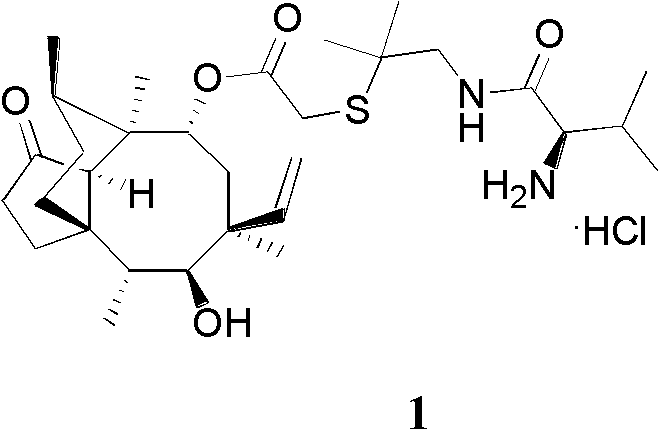
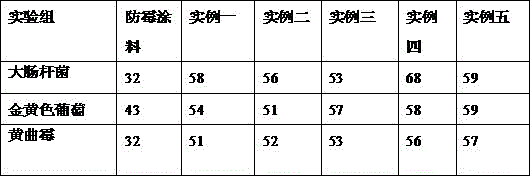
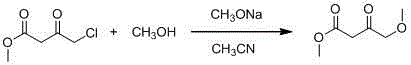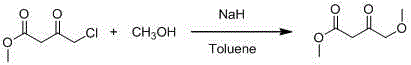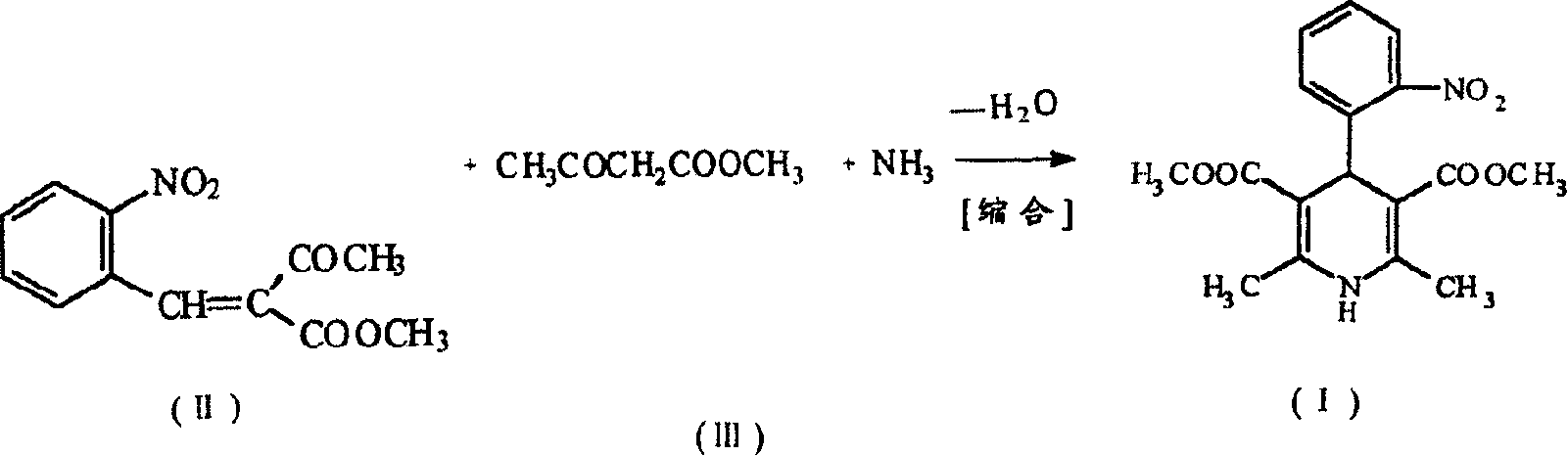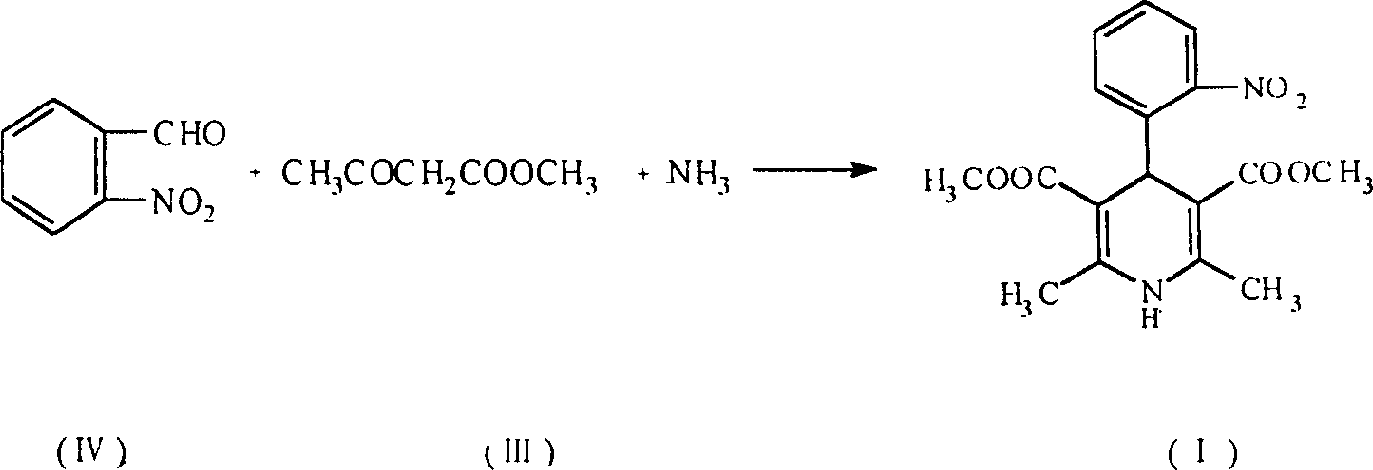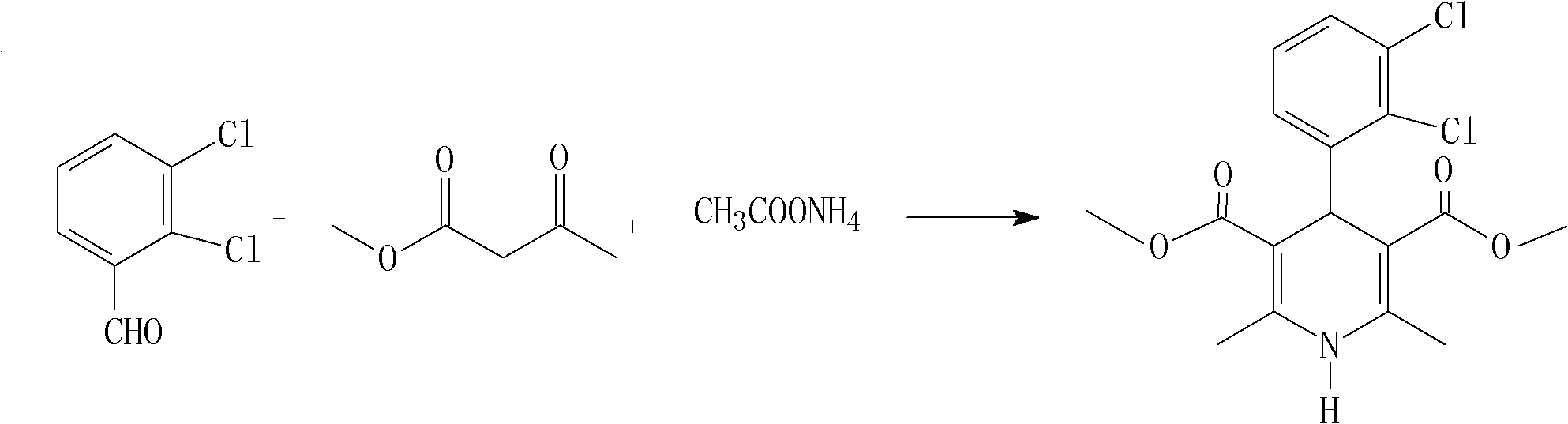Patents
Literature
152 results about "Methyl acetoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
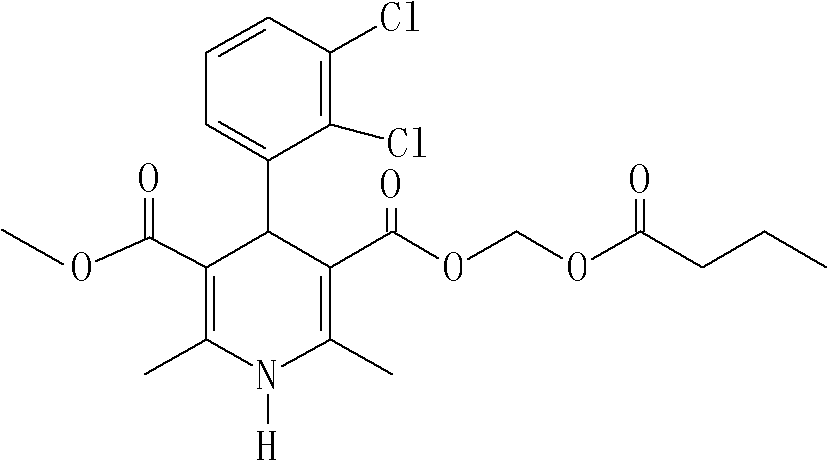
Method for preparing butyrate clevidipine
Owner:SUN YAT SEN UNIV +1
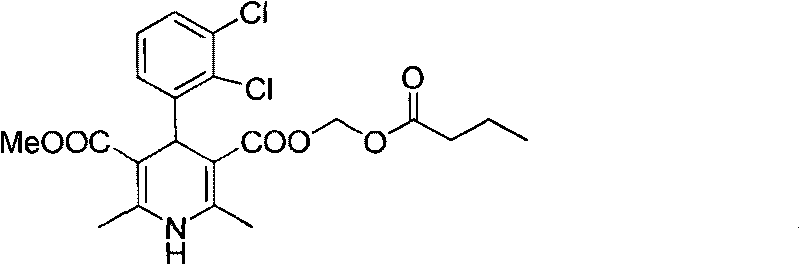
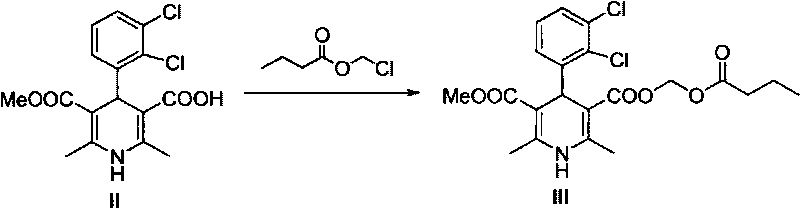
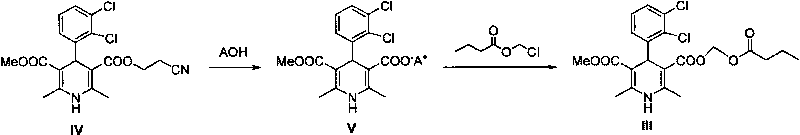
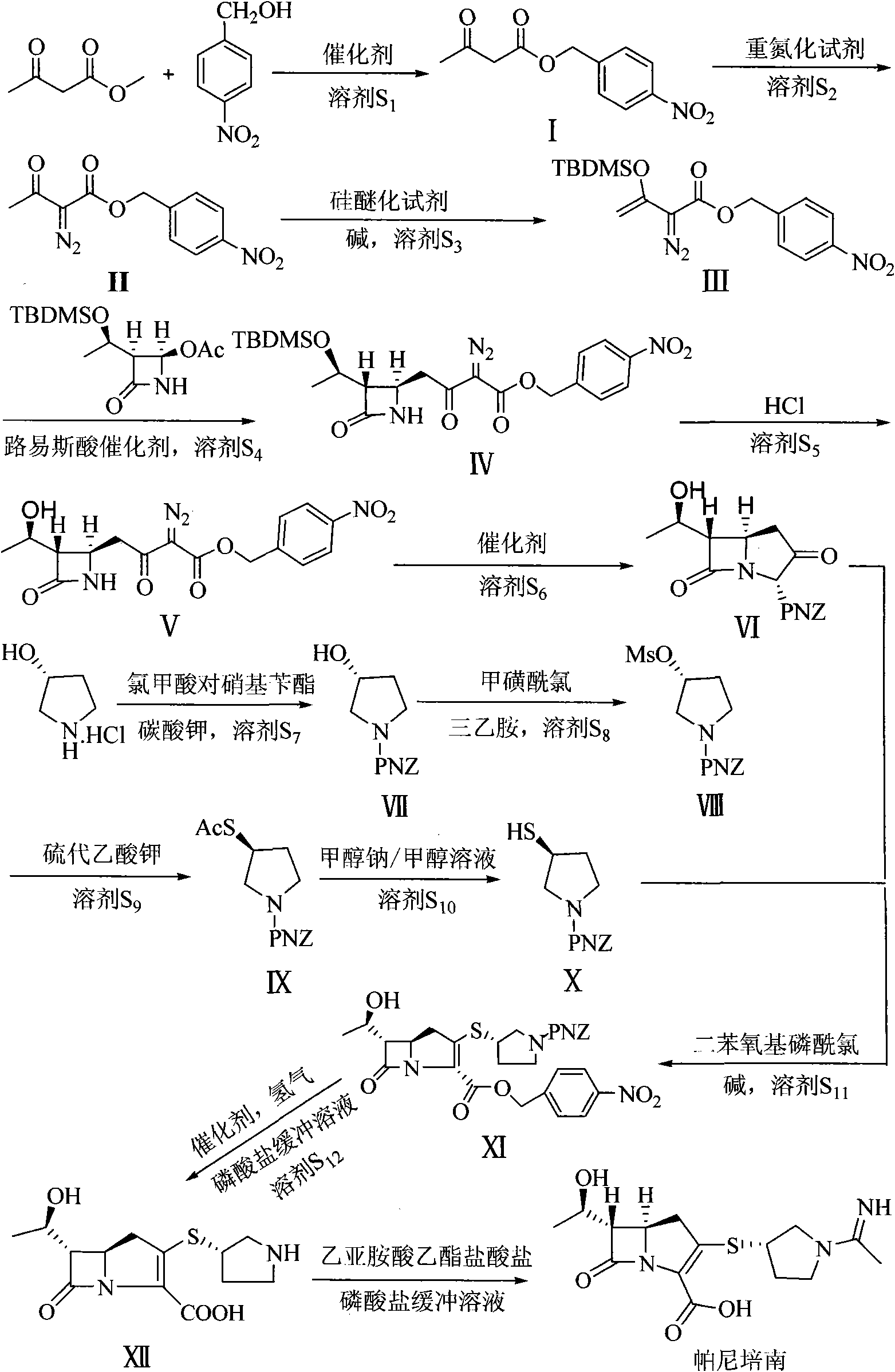
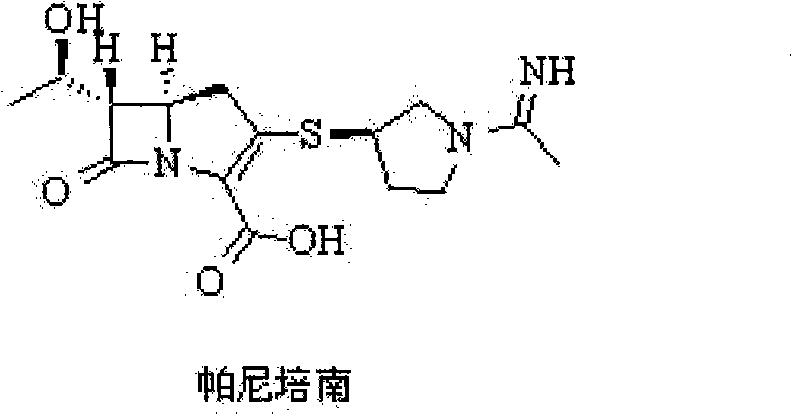
Preparation method of panipenem
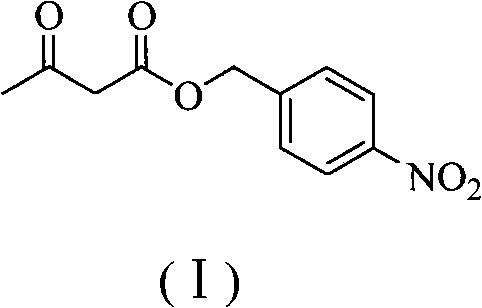
The invention relates to a preparation method of panipenem. Firstly, methyl acetoacetate and p-nitrobenzyl alcohol are taken as raw materials to prepare panipenem parent nucleus by six steps of reactions: ester exchange reaction, diazo reaction, enolization reaction, substitution reaction, hydrolysis reaction and ring closing reaction; then, (3R)-3-hydroxy-pyrrolidine hydrochloride, nitrobenzyl chroformate ester are taken as raw materials to prepare panipenem side chain by amidation reaction, sulfonylation reaction, nucleophilic substitution reaction and saponification reaction; finally, the prepared panipenem parent nucleus and panipenem side chain are in butt joint by condensation and are subjected to catalytic hydrolysis and imidization to obtain panipenem. The preparation method of panipenem features simple operation, mild reaction condition, friendly environment, high yield, good product purity, low cost and better industrialized production prospect.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method for preparing methyl acetoacetate by using novel composite catalyst
ActiveCN101337890AReduce energy consumptionIncrease production capacityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidBoiling point
The invention discloses a method for preparing methyl acetoacetate by using complex catalyst, which comprises the following steps: carrying out esterification reaction of methanol and diketene to generate crude methyl acetoacetate, wherein two different catalysts are added in the different stages of the esterification reaction; triethylenediamine catalyst is added before the esterification reaction; and diketene can be added directly dropwise without being heated by vapor, thereby overcoming the disadvantage that diketene is added dropwise after heating methanol to the boiling point in the conventional process and reducing the energy consumption; and cooling the generated liquid to 40 DEG C after the reaction, adding concentrated sulfur acid catalyst, keeping the temperature for half an hour, filtering, continuously rectifying the filtrate, and separating to obtain fine methyl acetoacetate with the content larger than 99%. Compared with the conventional intermittent rectification method, the continuous rectification method has greatly improved throughput and more convenient operation.
Owner:安徽天成新材料有限公司
A kind of production method of methyl acetoacetate
ActiveCN102276464AHigh purityThe four-column continuous negative pressure rectification of the high boiling column obtains a high purity with a content of 99.7%Preparation from ketenes/polyketenesAcetic acidEsterification reaction
The invention relates to a method for producing methyl acetoacetate. The method is characterized by comprising the following steps of: (1) performing esterification reaction of crude diketene and methanol at the temperature of between 20 and 150 DEG C, wherein in the esterification reaction, one of tertiary amine, an ethylidene-amine-containing compound or an alkaline compound is used as a catalyst; and (2) rectifying a product generated in the esterification reaction in the step (1) continuously in four towers under negative pressure to prepare methyl acetoacetate. In the method, the esterification reaction of the crude diketene is adopted, and the diketene is not needed to be rectified and purified, so energy consumption is low, the yield is high, the catalyst is easy to obtain, reaction conditions are mild, the process is safe and a product has high content and the yield of the product is high, and the method is suitable for large-scale industrial production.
Owner:NANTONG ACETIC ACID CHEM
New preparation method of key intermediate of clevidipine butyrate
InactiveCN101602710AShort reaction stepsMild reaction conditionsOrganic chemistryAcetic acidClevidipine
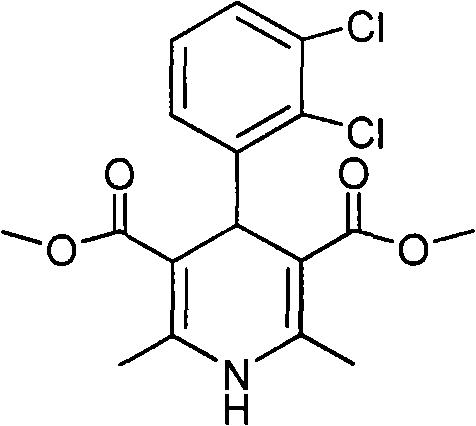
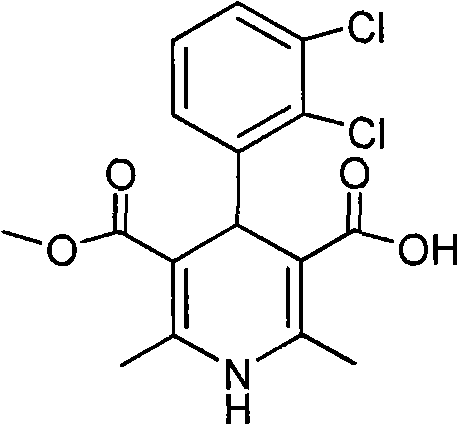
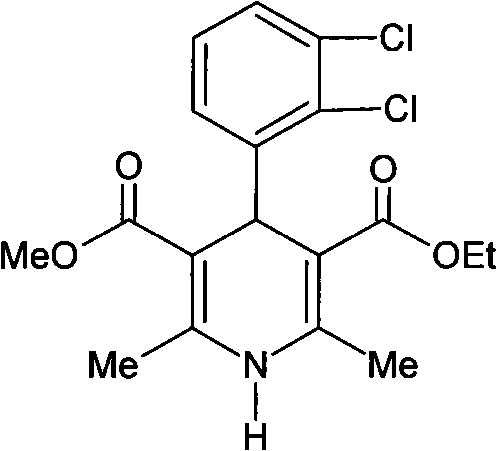
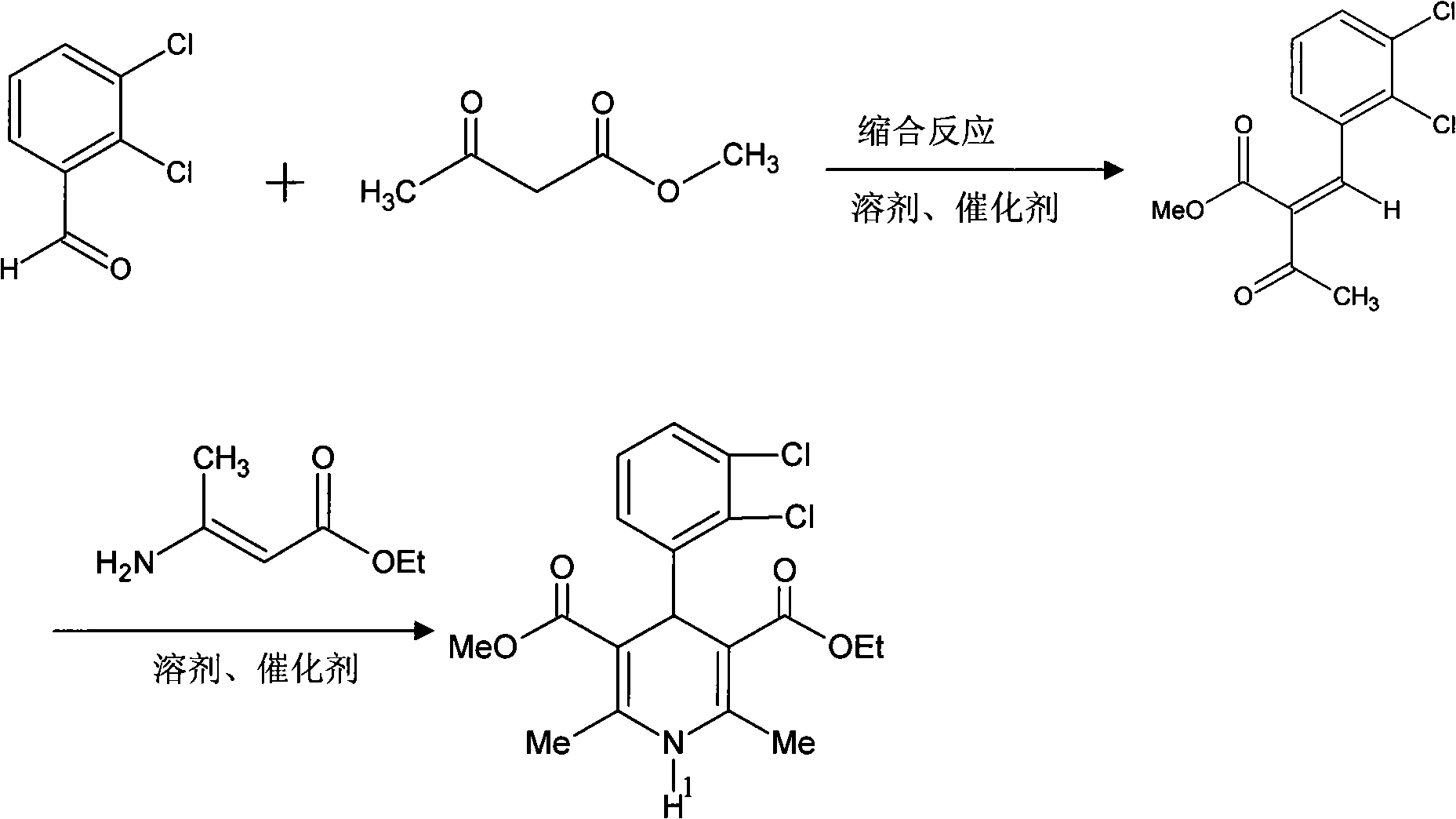
The invention relates to a preparation method of 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method is characterized in that methyl acetoacetate, stronger ammonia water and 2, 3-dichlorobenzaldehyde are used as raw materials for direct cyclic condensation to obtain 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-3, 5-pyridinedicarboxylic acid methyl ester (II); part of the compound II is hydrolyzed under alkaline condition to obtain the product 4-(2, 3-dichlorophenyl)-2, 6-dimethyl-1, 4-dihydro-5-methoxylcarbonyl-3-pyridinecarboxylic acid (I). The method has the advantages that the starting materials are cheap and easy to obtain, the production cost is lowered, the reaction is easy to operate and industrialization is easy to realize.
Owner:CHINA PHARM UNIV
Process for preparing amlodipine benzenesulphonate
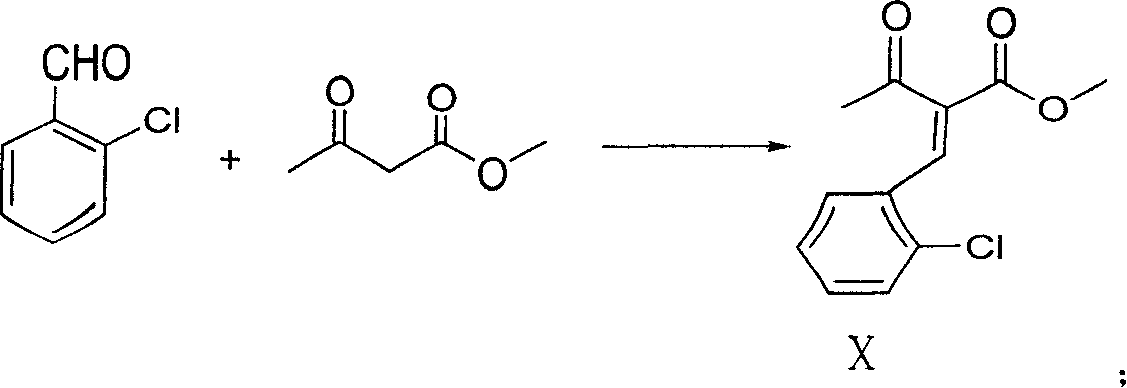
InactiveCN1927837ALow costShort reaction pathOrganic chemistryBulk chemical productionBenzaldehydeAmlodipine
The present invention discloses preparation process of Amlodipine benzene sulfonate. The preparation process includes the following steps: 1. the reaction between 4-[2-(tritylamindo)ethoxy] ethyl acetoacetate and amine compound to produce 3-amino-4-[2-( tritylamindo)ethoxy] ethylcrotonate; 2. the reaction between o-chluoro benzaldehyde and methyl acetoacetate under the catalysis of alkali to produce 2-(2-o-chluorobenzal)-ethyl acetoacetate; 3. the reaction of the products in the foregoing steps to obtain 4-(2-chlorophenyl)-6-methyl-2-((2-(tritylamido) ethoxy) methyl)-1, 4-dihydropyridyl-3-ethyl formate-5-methyl formate; and 4. further direct reaction with benzene sulfonic acid to eliminate protecting group and form Amlodipine benzene sulfonate.
Owner:上海开特生物科技有限公司
Method for preparing alumina aerogel
InactiveCN108328635AHigh densityHigh strengthAluminium oxide/hydroxide preparationSupercritical dryingAluminum Ion
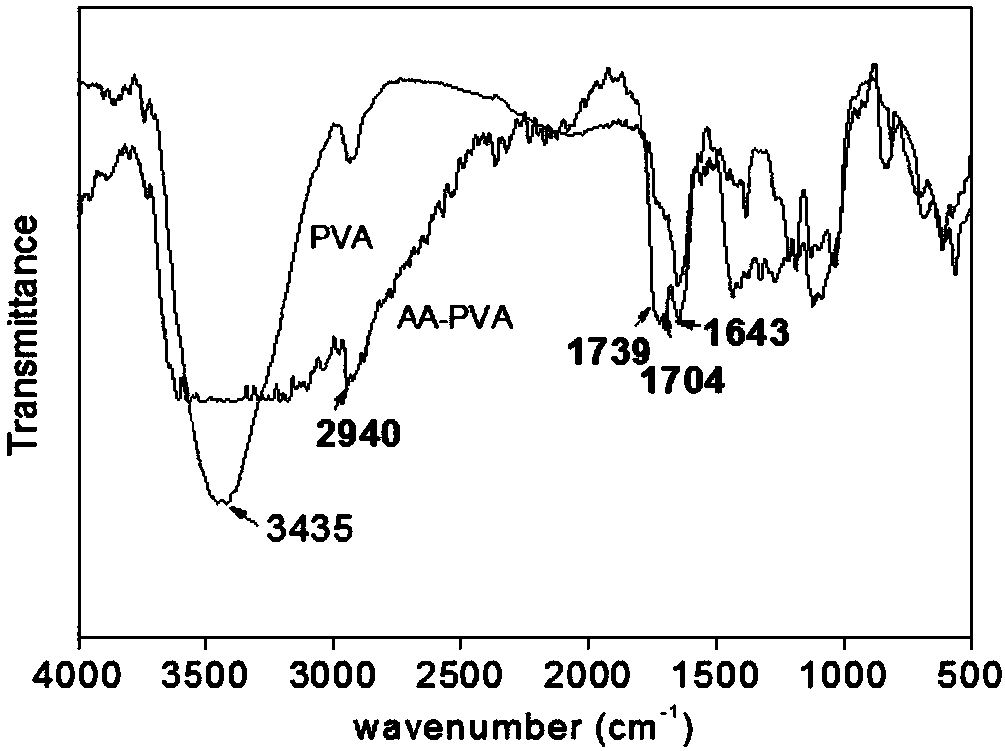
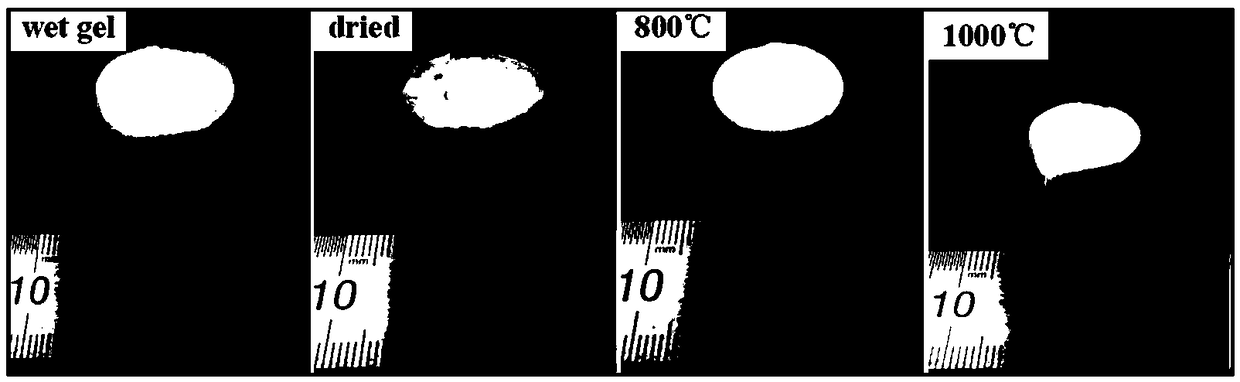
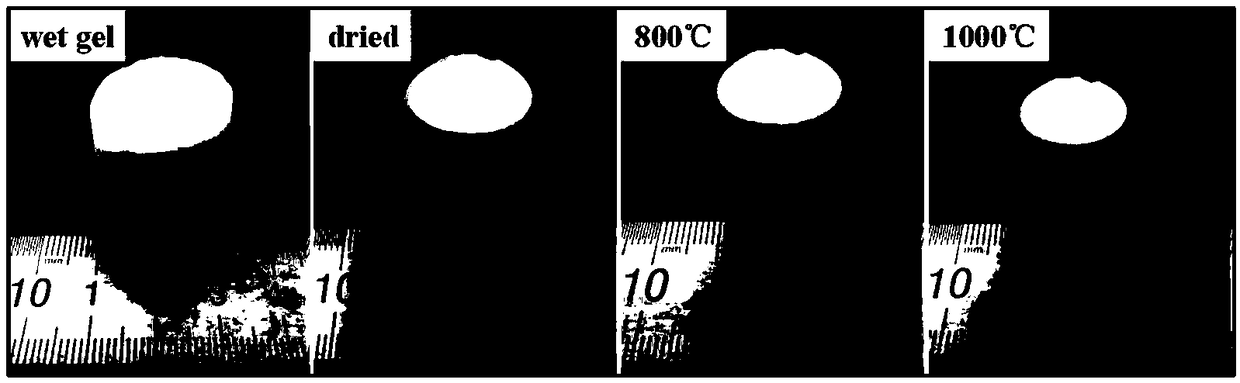
The invention relates to a method for preparing alumina aerogel. Firstly, methyl acetoacetate and polyvinyl alcohol are used as raw materials; concentrated sulfuric acid is used as a catalyst; throughester exchange reaction, acetoacetic acid groups are grafted on polyvinyl alcohol to prepare a macromolecule complexing agent; secondly, massive alumina aerogel with high density, high intensity, lowshrinkage rate, high porosity, high specific surface area and concentrated hole diameter distribution is obtained through preparation by using aluminium chloride hexahydrate as an inorganic phase precursor, using the macromolecule complexing agent as an additive, using deionized water as a solvent and using propylene oxide as a network gel inducting agent through the sol-gel process and the supercritical drying and roasting processes. The macromolecule complexing agent is added into the sol-gel process as a gel guiding agent and a dispersant, so that metal aluminum ion sol is uniformly dispersed in the solvent and is restrained by a macromolecule chain segment into a core; the primary particle aggregation mode is changed, so that the microstructure of the aerogel is changed. Meanwhile, the density and the intensity of the gel are enhanced.
Owner:SHANGHAI INST OF TECH
4-methoxy methyl acetoacetate preparation method
ActiveCN104478719AReduce usageReduce the introductionOrganic compound preparationCarboxylic acid esters separation/purificationDistillationInternal temperature
The invention discloses a 4-methoxy methyl acetoacetate preparation method. The 4-methoxy methyl acetoacetate preparation method comprises the following steps of adding a solvent tetrahydrofuran into a reaction kettle and leading inert gas into the reaction kettle, setting the internal temperature of the reaction kettle to be 15 DEG C to 25 DEG C, adding industrial sodium hydride and a metal alkaline compound in a stirring state, then adding the solvent tetrahydrofuran, dropwise adding mixed liquid of methyl alcohol and methyl-4-chloroacetoacetate at the temperature of lower than 20 DEG C to perform reaction for 4-6 hours, rising the temperature to be 20 DEG C to 25 DEG C to continue to react for 4-15 hours, reducing the system temperature to be 6 DEG C to 10 DEG C after TLC detection reaction is completed, adding a hydrochloric acid solution with molar concentration of 2 mol / L to regulate the pH of a system to be 5 to 7, performing standing and laying, concentrating and spin-drying upper-layer liquid to remove the solvent tetrahydrofuran after liquid separation and then obtaining a colorless product 4-methoxy methyl acetoacetate through wiped-film molecular distillation. The 4-methoxy methyl acetoacetate can react at room temperature, a product can be produced at low temperature through distillation, and dangerousness in the production process and product impurity content can be directly and effectively reduced.
Owner:ZHONGSHAN NIKEMEI PHARMA
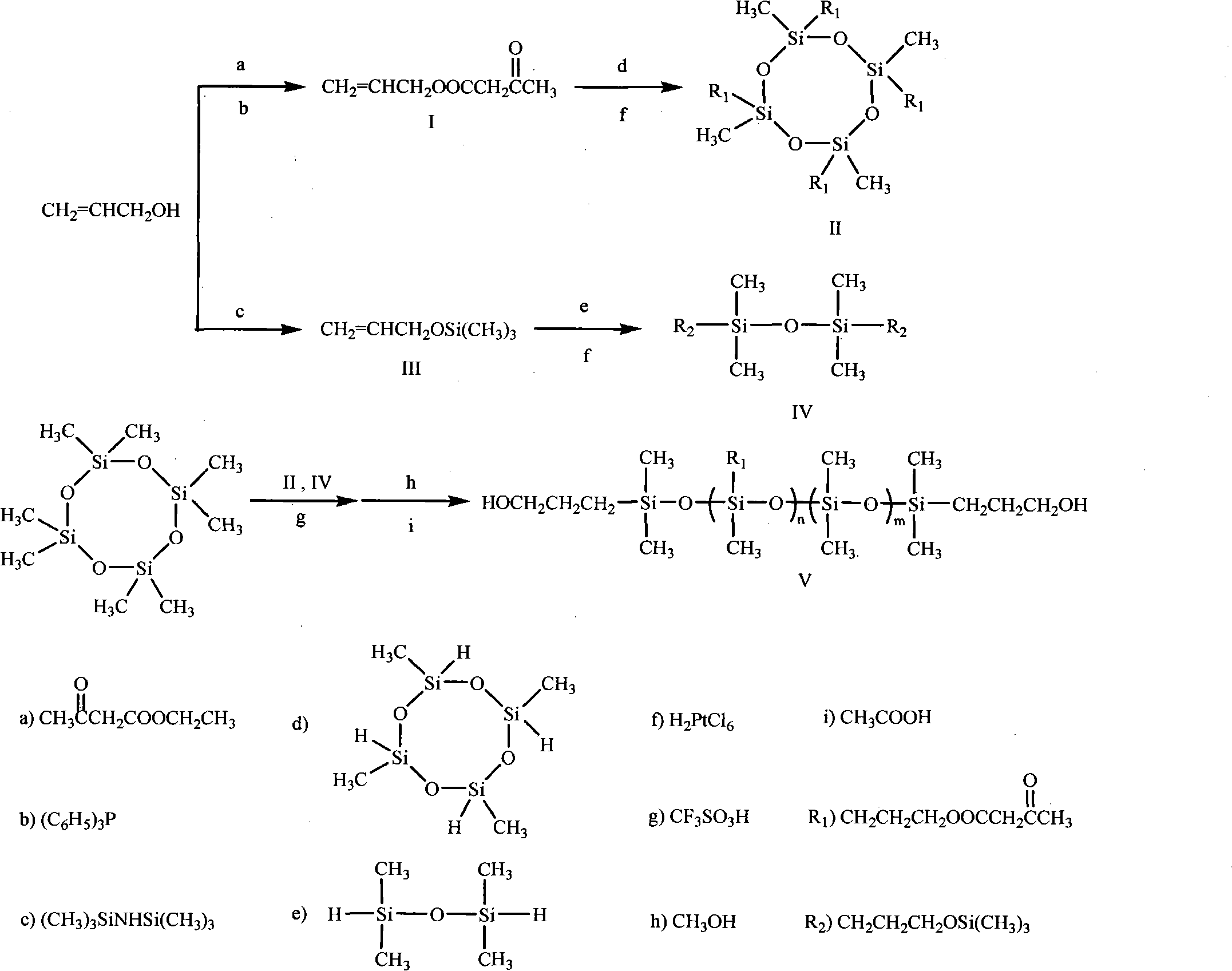
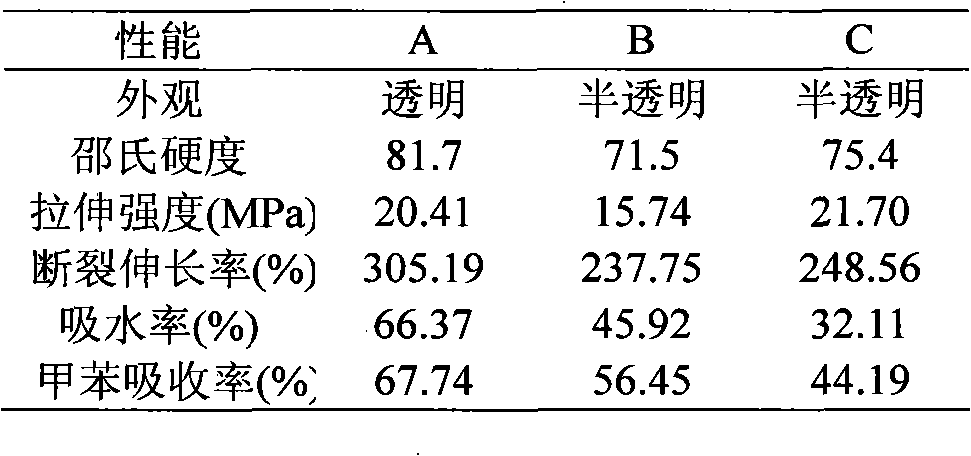
Method for synthesizing ketocarbonyl-containing bis-hydroxypropyl terminated polysiloxane
InactiveCN101885844AImprove water resistanceIncrease the relative molecular massPolyurea/polyurethane coatingsAcetic acidBis(trimethylsilyl)amine
The invention discloses a method for synthesizing ketocarbonyl-containing bis-hydroxypropyl terminated polysiloxane. A target compound, namely the bis-hydroxypropyl terminated polysiloxane of which the side group contains ketocarbonyl, is prepared by taking methyl acetoacetate (or ethyl acetoacetate), allyl alcohol, hexamethyldisilazane, tetramethyldisilazane, tetramethylcyclotetrasiloxane and octamethylcyclotetrasiloxane as starting materials, and by performing ester interchange, hydroxyl protection, hydrosilylation, equilibrium polymerization and alcoholysis reaction. The purity of the product is over 98 percent.
Owner:INST OF QUALITY STANDARDS & TESTING TECH FOR AGRO PROD OF SHANDONG ACADEMY OF AGRI SCI
Prepn process of nifedipine
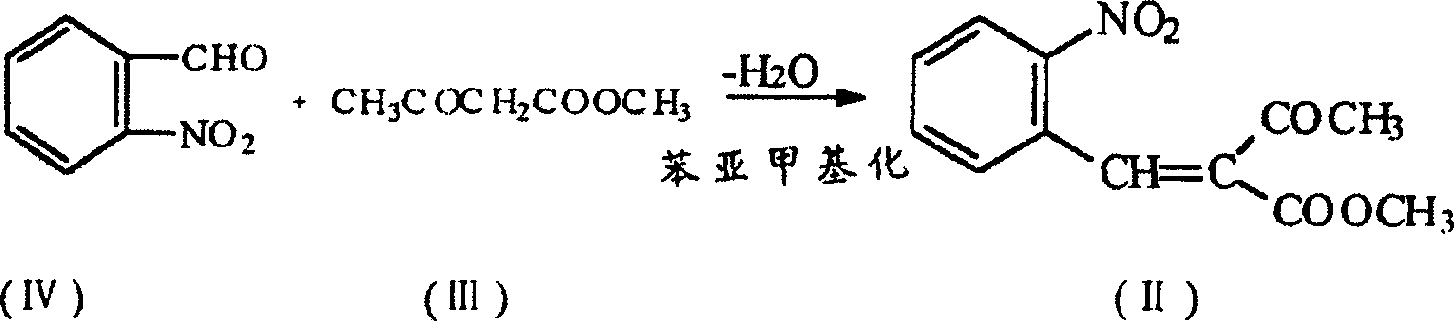
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde,the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
The preparation method of felodipine
InactiveCN102285911AEasy to operatePreparative route steps are short and effectiveOrganic chemistryAcetic acidEthyl ester
The invention discloses a method for preparing felodipine, which belongs to the technical field of felodipine synthesis. The method is characterized by comprising the following steps of: performing a Knoevenagel condensation reaction of 2,3-dichlorobenzaldehyde and methyl acetoacetate which are used as initial raw materials to obtain 2,3-dichlorobenzylidene acetoacetate; and performing a Michael cyclization reaction of 2,3-dichlorobenzylidene acetoacetate and 3-amino ethyl crotonate to obtain the felodipine to obtain the felodipine. The method has the advantages that the preparation route is simple, short and reliable; the preparation period is short; the method is simple and convenient to operate and has high safety and low environmental pollution; residual toxicity of a medicament is low; and the post treatment process has high efficiency.
Owner:SHAOXING UNIVERSITY
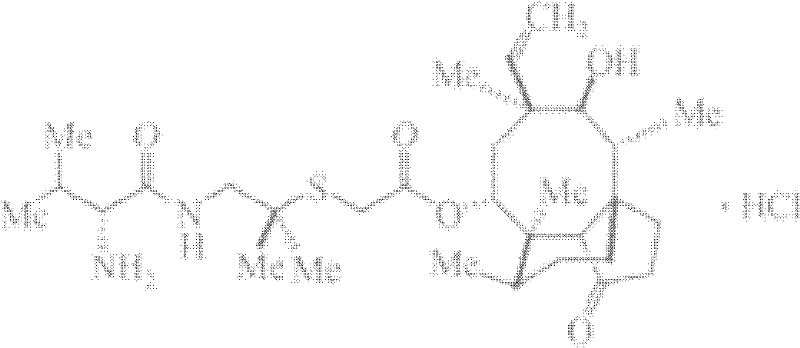
Chemical synthesis method of valnemulin hydrochloride
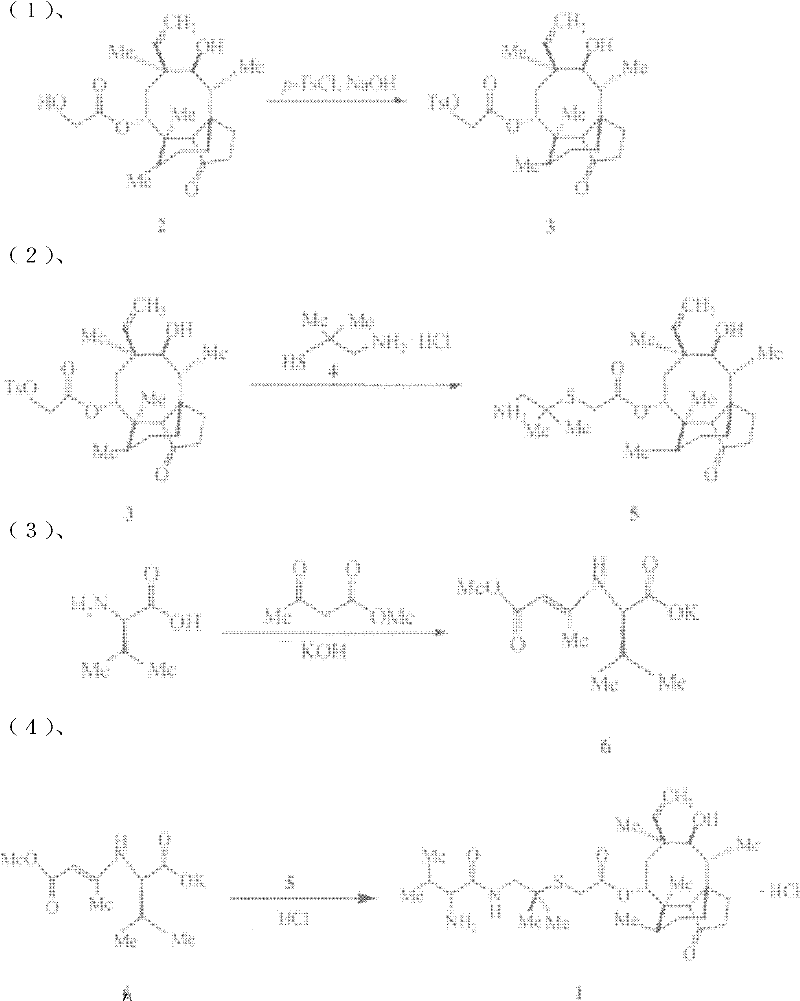
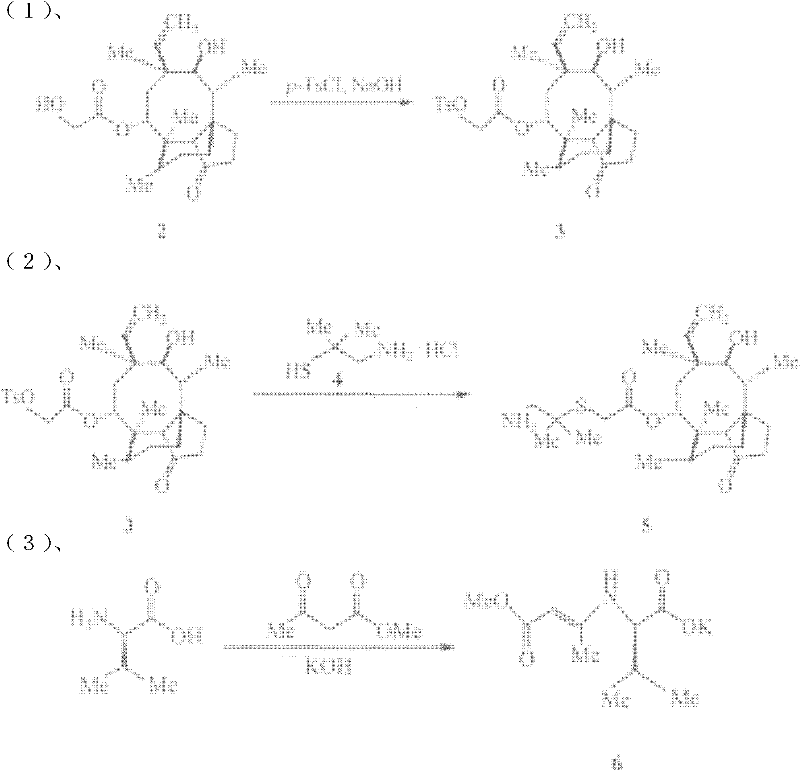
The invention discloses a chemical synthesis method of valnemulin hydrochloride, which comprises the following steps of: taking refined pleuromutilin as raw material, carrying out sulfonation by paratoluensulfonyl chloride, and reacting with dimethyl cysteamine hcl, to obtain the pleuromutilin dimethyl cysteamine substitute; reacting D-valine, methyl acetoacetate and potassium hydroxide to obtain (R)-2-(1-methoxycarbonyl group-2-allyl) amino-3-methyl potassium butyrate, activating by ethyl chloroformate and reacting with the pleuromutilin dimethyl cysteamine substitute, adjusting PH value, carrying out reverse phase extraction, and carrying out freeze-drying to obtain the valnemulin hydrochloride. The method has the advantages that due to the refining of the raw material pleuromutilin, the impurities in the product can be effectively removed, and the purifying process can be simplified from the source; the carboxyl of D-valine can be activated by the ethyl chloroformate, so that the reaction is easier to carry out; and due to the pH adjustment, the reverse phase extraction, and the freeze-drying, the product can be obtained, so that the product is stable in quality, and high in purity.
Owner:武汉回盛生物科技股份有限公司
Preparation method of methyl acetoacetate
ActiveCN103450017AImprove stabilityReduce consumptionPreparation from ketenes/polyketenesAcetic acidNitrate
The invention provides a preparation method of methyl acetoacetate. The preparation method sequentially comprises the following steps of: (1) fully dissolving methanol and a catalyst, then adding the mixture into an esterification reaction kettle, heating up, and dropwise adding ketene dimer; (2) after completing dropwise addition, insulating, and cooling to produce crude methyl acetoacetate; (3) rectifying the crude product to obtain the finished methyl acetoacetate. The preparation method is characterized in that the catalyst in the step (1) is an amine type ionic liquid catalyst which is selected from one or more of n-butylamine nitrate, n-butylamine acetate, ethylamine nitrate, ethylamine acetate, propylamine nitrate and propylamine acetate. According to the preparation method of methyl acetoacetate, the amine type ionic liquid catalyst is researched and is good in stability, the stability of methyl acetoacetate is enhanced under the action of the catalyst, the rectification yield of a product can be improved, the technological operations are simple, the raw material consumption is reduced, and the catalyst has obvious advantages and a positive effect.
Owner:NANTONG ACETIC ACID CHEM
Preparation method of felodipine synthetic intermediate methyl 2-(2,3-dichlorobenzylidine)acetoacetate
ActiveCN101613280AHigh purityGood crystal formOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical structureAcetic acid
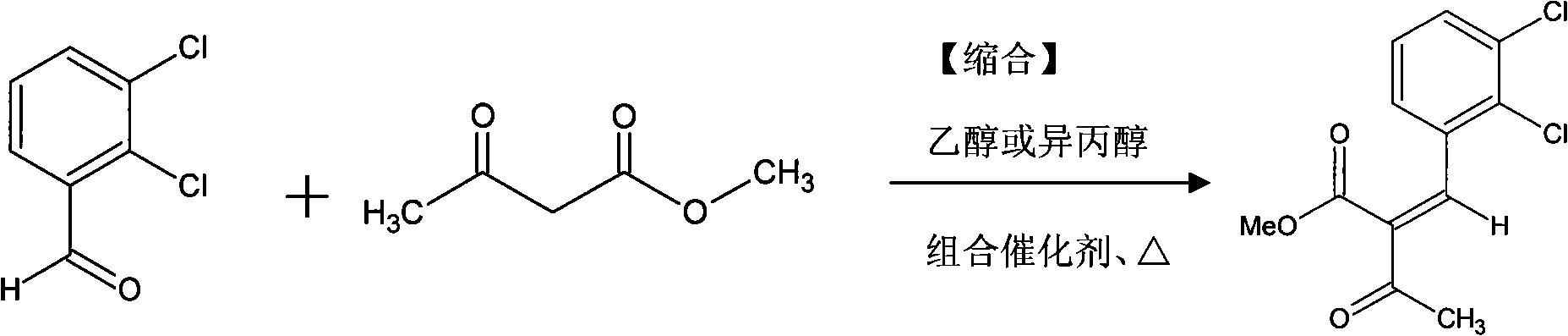
The invention discloses a preparation method of methyl 2-(2,3-dichlorobenzylidine)acetoacetate which is a synthetic intermediate of antihypertensive drug felodipine. The method is characterized by allowing a condensation reaction between 2, 3-dichlorobenzaldehyde and methyl acetoacetate in alcohol solution under the catalytic action of a novel combined catalyst consisting of secondary amine and quinoline carboxylic acid. Reaction liquid is cooled at room temperature for crystallization, filtered, washed and dried after the condensation reaction to obtain primary eluent of the intermediate methyl 2-(2,3-dichlorobenzylidine)acetoacetate with purity not less than 98%. Mother liquor is subject to vacuum condensation, cooling crystallization, filtering and recrystallization to obtain secondary eluent product of the intermediate methyl 2-(2,3-dichlorobenzylidine)acetoacetate with purity not less than 98%. Compared with the prior art, the new method helps enhance synthetic yield of the felodipine intermediate, and provide ideal intermediate purity. The method is also applicable to preparing other 4-sustituted-1,4-dihydropyridine antihypertensive drug intermediates with chemical structure and pharmaceutical and clinical actions similar to those of felodipine.
Owner:HEFEI LIFEON PHARMA
Prepn. process of nifedipine
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde, the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
Preparation method of valnemulin hydrochloride
InactiveCN102225905AReduce pollutionMild reaction temperatureSulfide preparationReaction temperatureSolvent
The invention relates to a preparation method of valnemulin hydrochloride. The preparation method comprises the following steps: reacting pleuromutilin with p-toluenesulfonyl chloride, substituting with 1-amino-2-methyl propyl-2-mercaptan hydrochloride so as to obtain [(2-amino-1,1-dimethyl S, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester for later use; reacting D-valine with methyl acetoacetate; synthesizing the product with isobutyl chloroacetate so as to generate anhydride; and reacting anhydride with [(2-amino-1,1-dimethyl ethyl) sulfenyl] acetic acid (3aS, 4R, 5, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester so as to generate amide, and then carrying out deprotection with hydrochloric acid so as to prepare valnemulin hydrochloride. The method has the advantages that (1) reaction temperature is mild, thereby being applicable to large-scale production; (2) post-treatment is simple, thereby directly obtaining the product; (3) used solvents are less, thereby reducing environmental pollution; and (4) the valnemulin hydrochloride content and yield of the obtained product are high.
Owner:HUBEI SHENZHOU CHEM
Preparing method of flame-retardant, anti-bacterial and waterproof graphene modified acrylate resin paint
ActiveCN105820695AEasy to useLight colorFireproof paintsAntifouling/underwater paintsAcetic acidAcrylic resin
The invention provides a preparing method of flame-retardant, anti-bacterial and waterproof graphene modified acrylate resin paint .After being stripped, walnut green peels are cleaned with water, placed into an oven to be dried at the temperature of 40-70 DEG C to the constant weight, smashed and screened through 80-100 meshes to obtain walnut green peel powder, the walnut green peel powder and water are weighed, allyl trimethyl ammonium chloride and 4-methoxy methyl acetoacetate are added, the mixture is subjected to backflow decoction at the temperature of 80-100 DEG C, decoction liquid is filtered and subjected to pressure-reduction concentration at the temperature of 60-70 DEG C, and walnut green peel extract concentrate is obtained after the decoction liquid is concentrated into dense paste with the water content being 15-20%, and applied to acrylic resin .The obtained acrylic resin has good anti-bacterial, flame-retardant and waterproof properties .
Owner:河北虹阳金盈建材有限公司
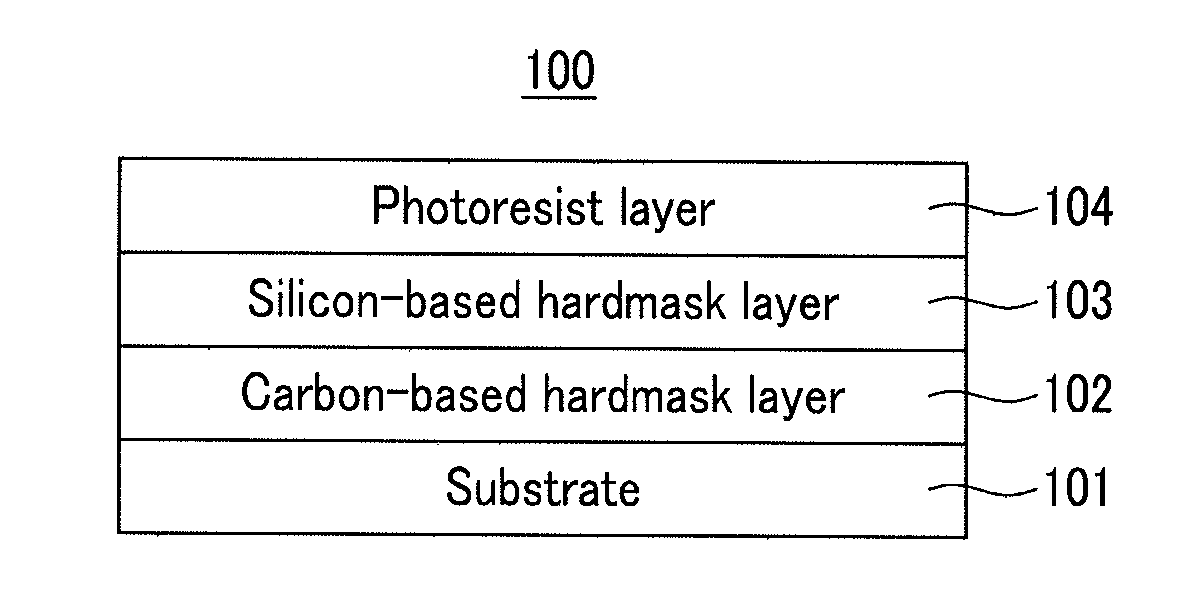
Hardmask composition for forming resist underlayer film, process for producing a semiconductor integrated circuit device, and semiconductor integrated circuit device
InactiveUS20110241175A1Good coating performanceExquisite patternPhotomechanical apparatusSemiconductor/solid-state device manufacturingResistButyric anhydride
A hardmask composition for forming a resist underlayer film, a process for producing a semiconductor integrated circuit device, and a semiconductor integrated circuit device, the hardmask composition including an organosilane polymer, and a stabilizer, the stabilizer including one of acetic anhydride, methyl acetoacetate, propionic anhydride, ethyl-2-ethylacetoacetate, butyric anhydride, ethyl-2-ethylacetoacetate, valeric anhydride, 2-methylbutyric anhydride, nonanol, decanol, undecanol, dodecanol, propylene glycol propyl ether, propylene glycol ethyl ether, propylene glycol methyl ether, propylene glycol, phenyltrimethoxysilane, diphenylhexamethoxydisiloxane, diphenylhexaethoxydisiloxane, dioctyltetramethyldisiloxane, hexamethyltrisiloxane, tetramethyldisiloxane, decamethyltetrasiloxane, dodecamethylpentasiloxane, hexamethyldisiloxane, and mixtures thereof.
Owner:CHEIL IND INC
Printing ink composition
InactiveUS20090062473A1Good physical propertiesGood effectFlexible coversWrappersCross-linkAcetoacetates
Disclosed is a printing ink composition containing, as a cross-linking agent, a metal complex including a β-ketoester, for example, an acetoacetate ester such as methyl acetoacetate or ethyl acetoacetate, as a ligand; and a maleic acid resin.
Owner:DAINIPPON INK & CHEM INC
Method for preparing clevidipine butyrate
ActiveCN102432527AHigh selectivityReduce generationOrganic chemistryFermentationClevidipineCarboxylic acid
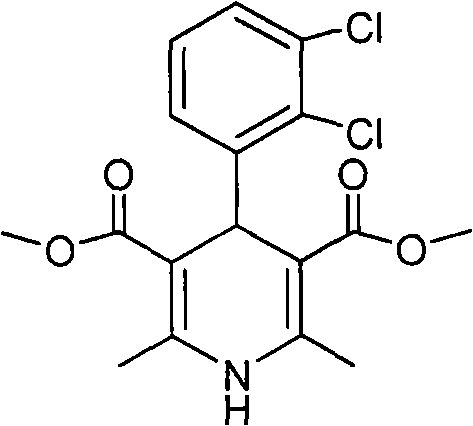
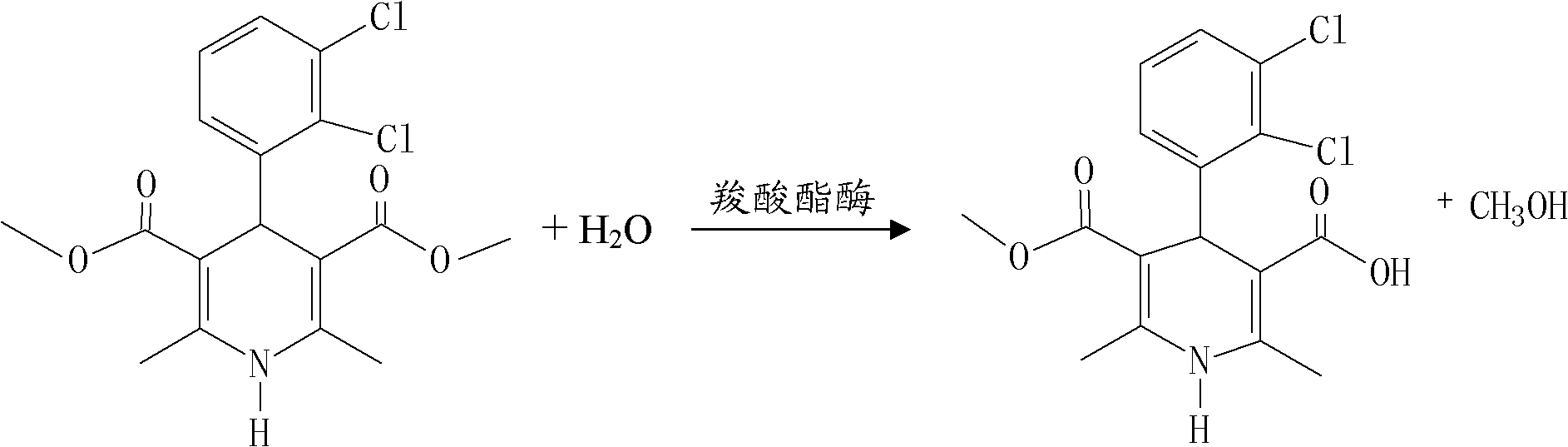
The invention relates to a method for preparing clevidipine butyrate and also relates to a method for preparing 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-methoxycarbonyl-3-picolinic acid methyl ester (an intermediate I) and 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-methoxycarbonyl-3-pyridine carboxylic acid (an intermediate II) which are used as intermediates of the clevidipine butyrate. The intermediate I is prepared by using 2,3-dichlorobenzaldehyde, ammonium acetate and methyl acetoacetate as raw materials and performing a reaction by using ultrasound. The intermediate II is prepared by carrying out enzyme hydrolysis on the intermediate I. When the clevidipine butyrate is prepared by using the method disclosed by the invention, ammonia water is not used as the raw material, so that no irritant gas is generated. Moreover, the cyclizative condensation is carried out by using ultrasonic waves and the hydrolysis is carried out by using carboxy lesterase, so that the reaction time can be shortened and the reaction yield is increased.
Owner:ZHEJIANG JIUXU PHARMA
Method for continuously producing methyl acetoacetate by using micro-channel reactor
InactiveCN111039785AExtended service lifeShort reaction timePreparation from ketenes/polyketenesWater methanolPtru catalyst
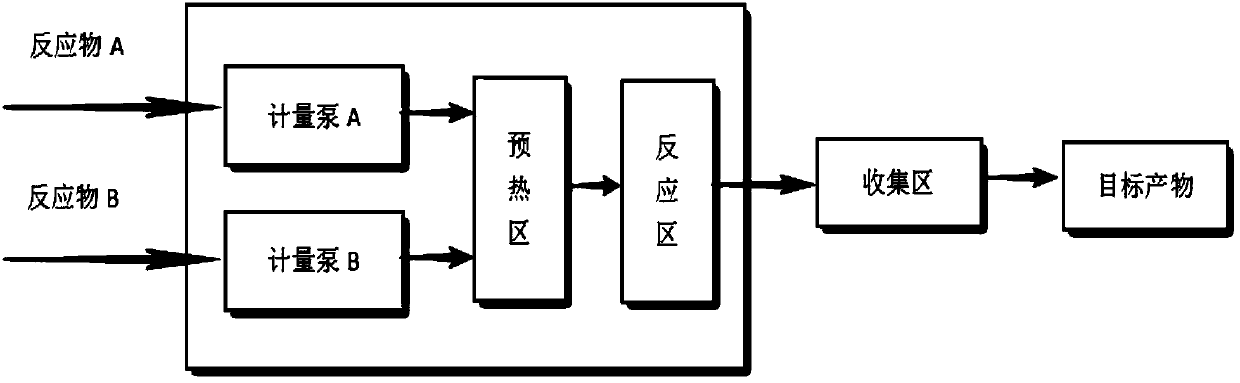
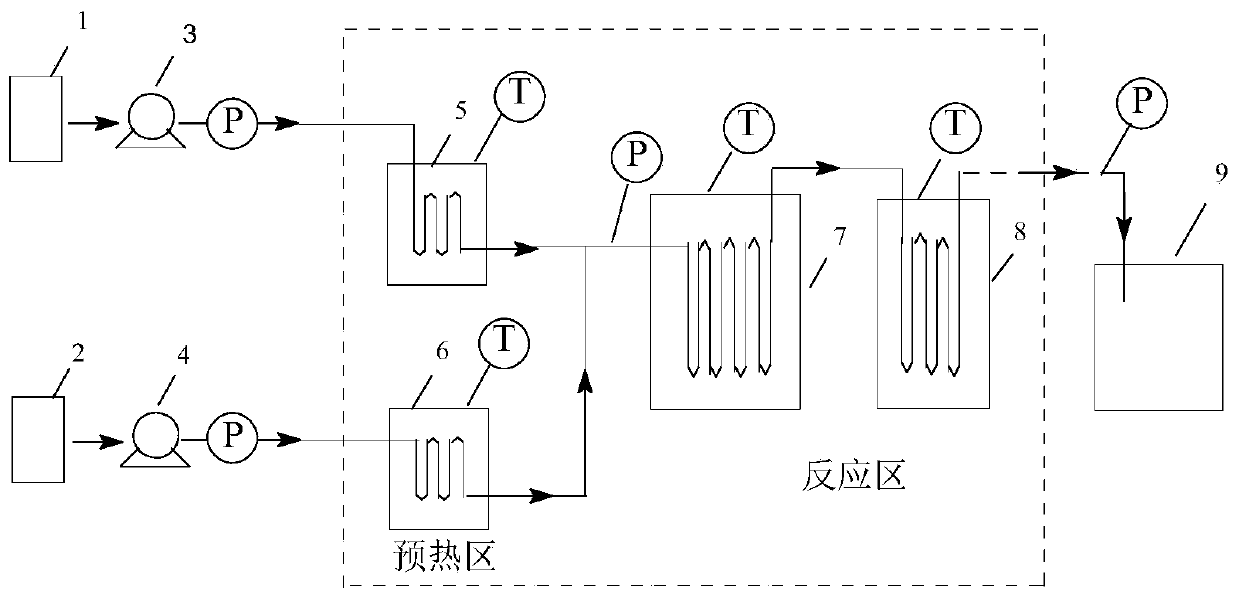
The invention discloses a method for continuously producing methyl acetoacetate by using a micro-channel reactor, and belongs to the technical field of organic synthesis processes. Diketene and absolute methanol are used as raw materials, an acid or alkali is used as a catalyst, and the preparation process of methyl acetoacetate is continuously completed in a micro-channel reactor system. According to the method, after materials are fed into a micro-channel reactor through metering pumps, preheating, mixing, reacting and separation are performed to obtain a methyl acetoacetate product. In themethod, the temperature and the residence time during the reaction process can be strictly controlled, the reaction temperature can be accurately controlled so as to prevent temperature runaway, and the safety of the reaction apparatus can be improved; due to the strong mass transfer effect of the micro-channel reactor, the mass transfer effect among the raw materials in the reaction system is enhanced, and the reaction efficiency is greatly improved.
Owner:CHANGZHOU UNIV
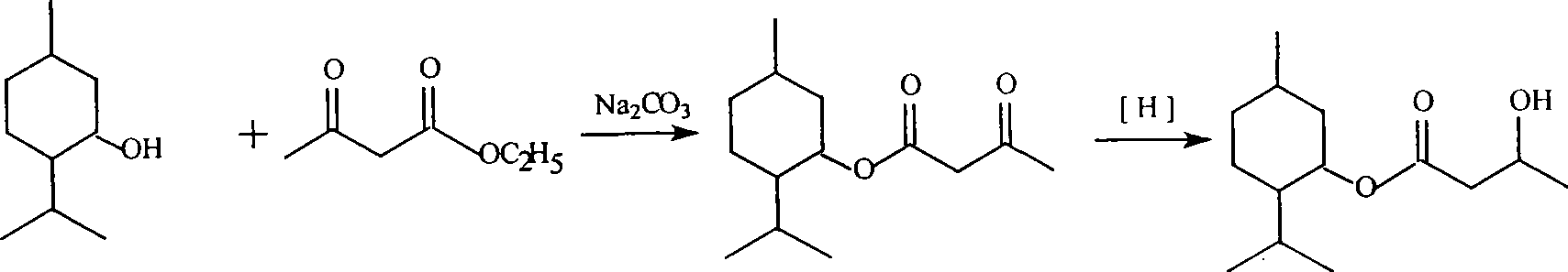
Method for synthesizing cooling agent L-menthyl 3-hydroxybutyrate
InactiveCN101423474ARaw materials are easy to getMild conditionsPreparation by ester-hydroxy reactionReaction temperatureSolvent
The invention discloses a synthetic method for 3-hydroxybutyric acid L-mint ester. The synthetic method comprises two steps: a first step, namely, a synthetic method for acetoacetic acid L-mint ester, which is characterized in that acetoacetic ester or methyl acetoacetate and L-mint camphor are subjected to ester exchange reaction under the action of a basic catalyst to prepare the acetoacetic acid L-mint ester, wherein the reaction time is 4 to 5 hours, the catalyst used in the reaction is sodium carbonate or potassium carbonate or calcium carbonate, and solvent used in the reaction is methyl benzene or benzene or cyclohexane; and a second step, namely, the acetoacetic acid L-mint ester is reduced to obtain the 3-hydroxybutyric acid L-mint ester, wherein the reaction temperature is between 10 and 20 DEG C, the solvent used in the reaction is methanol or ethanol, and a reducer in the reaction is potassium borohydride or sodium borohydride. The synthetic method has the advantages of easily obtained raw materials, mild conditions, simple and convenient operation, and high yield.
Owner:上海香料研究所
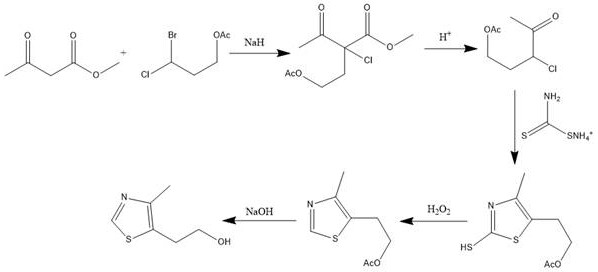
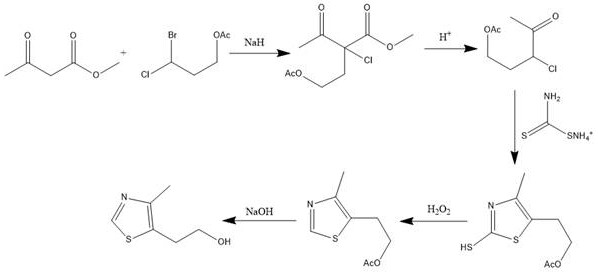
Synthetic method of 5-(2-hydroxyethyl)-4-methylthiazole
PendingCN111635375AFew reaction stepsShort reaction timeOrganic chemistryOrganic synthesisMethyl palmoxirate
The invention belongs to the field of organic synthesis, and discloses a 5-(2-hydroxyethyl)-4-methylthiazole synthesis method, which comprises: preparing 4-acetoxy-2-acetyl-2-methyl chlorobutyrate from methyl acetoacetate and 3-bromo-3-chloropropyl acetate under an alkaline condition; hydrolyzing the 4-acetoxy-2-acetyl-2-methyl chlorobutyrate under an acidic condition to prepare 3-chloro-3-acetylpropanol acetate; preparing 2-mercapto-4-methyl-5-(beta-acetoxyethyl)-thiazole from the 3-chloro-3-acetyl propanol acetate and ammonium dithiocarbamate under an acidic condition; adding an oxidizing agent into the 2-mercapto-4-methyl-5-(beta-acetoxyethyl)-thiazole under an acidic condition to prepare 4-methyl-5-(beta-acetoxyethyl)-thiazole; and preparing 4-methyl-5-(beta-acetoxyethyl)-thiazole from the 4-methyl-5-(beta-acetoxyethyl)-thiazole under an alkaline condition. The synthesis method is mild in overall reaction condition, simple in post-treatment and suitable for pilot scale test and industrial production.
Owner:KUNSHAN YAXIANG SPICEL CO LTD
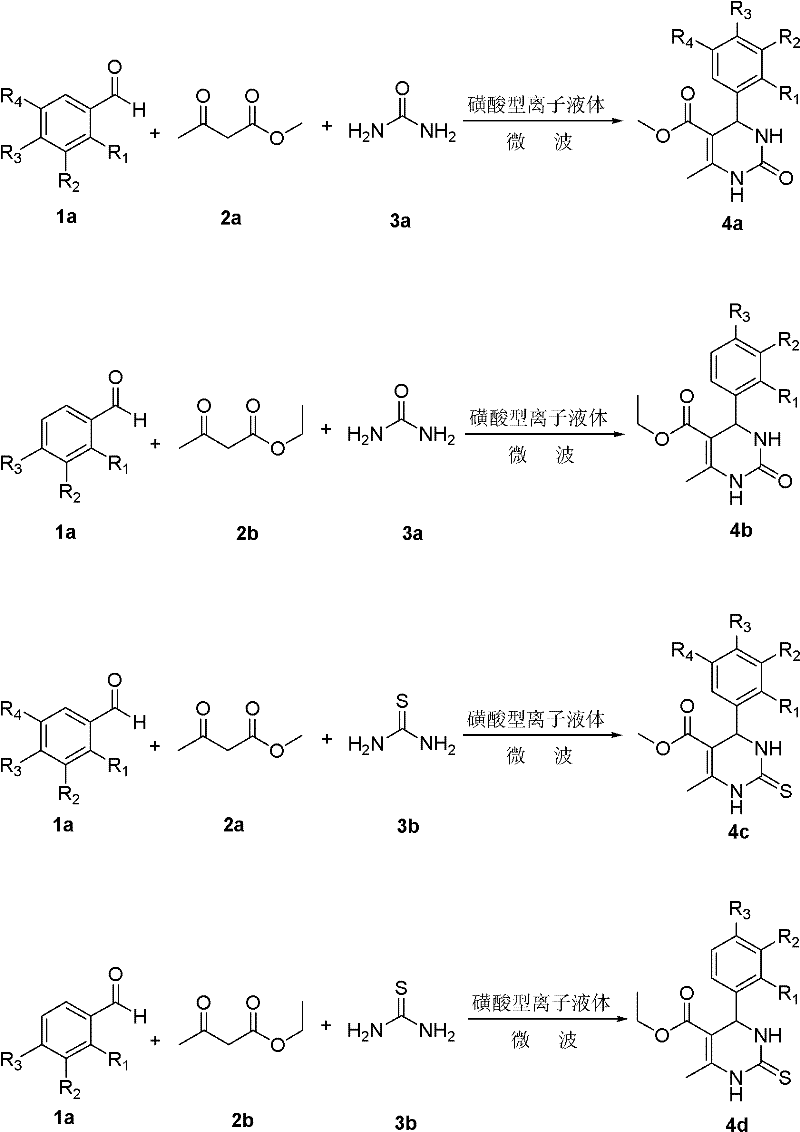
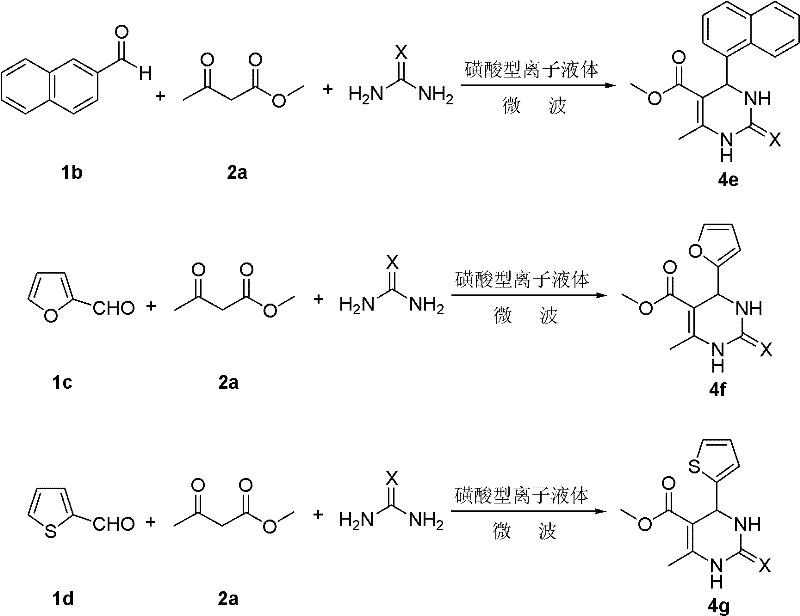
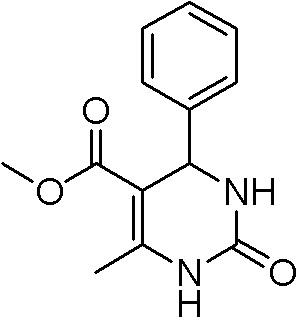
Environmentally-friendly synthetic method of pyrimidine compound
The invention relates to a synthetic method of a pyrimidine derivative. In the method, a sulphonic acid ion liquid is taken as a catalyst, aromatic aldehyde, methyl acetoacetate (ethyl ester) and urea (or thiourea) are taken as raw materials, and a reaction is undergone under the action of microwave irradiation under a solvent-free condition. The molar ratio of aldehyde serving as a reactant to ester serving as a reactant to urea serving as a reactant (or thiourea) is 1:(0.5-2):(0.5-2); the ratio of the sulphonic acid ion liquid to the aldehyde is 1:(10-50); the reaction time is 10-50 minutes; the reaction temperature is 50-120 DEG C; and the pressure is 1-1.3 MPa. The synthetic method has the characteristics of easiness in operation, readily-available raw materials, small number of reaction steps, high yield, reusable catalyst, environmental friendliness, and the like.
Owner:TAIZHOU UNIV
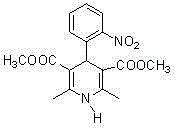
Preparation method of methyl 2-nitrobenzal acetoacetate
ActiveCN102976949AHigh purityMild reaction conditionsOrganic chemistryOrganic compound preparationNifedipineAcetic acid
The invention provides a preparation method of methyl 2-nitrobenzal acetoacetate, which comprises the following steps: (1) reacting initial raw materials o-nitrobenzaldehyde and methyl acetoacetate with methanol under the action of a catalyst to generate a compound methyl 2-nitrobenzal acetoacetate; (2) after carrying out vacuum concentration to remove the back, adding a crystallizing solvent, and stirring at low temperature to generate the methyl 2-nitrobenzal acetoacetate solid crude product; and (3) recrystallizing the solid crude product to obtain the methyl 2-nitrobenzal acetoacetate pure product of which the purity is higher than 99.5%. The preparation method provided by the invention has the advantages of mild reaction conditions, short reaction time, high yield and environmental protection; the obtained nifedipine has high purity of the impurity methyl 2-nitrobenzal acetoacetate; and the impurity can be accurately positioned by comparison in the nifedipine related substance inspection, thereby having important instruction meanings for researching nifedipine.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Softener for bamboo weaving
The invention discloses a softener for bamboo weaving. The softener comprises raw material components in parts as follows: 50-60 parts of triethanolamine, 30-40 parts of aluminum stearate, 40-50 parts of barium stearate, 40-50 parts of paraffin wax, 30-35 parts of polyethylene glycol, 30-35 parts of acetone, 10-15 parts of hexamethylene diisocyanate, 10-12 parts of methyl acetoacetate, 8-10 parts of malonic acid diethyl ester, 6-8 parts of acetoacetic acid, 5-8 parts of chlorinated paraffin, 6-10 parts of xylene, 5-6 parts of glycerinum, 6-8 parts of ethylparaben, 3-5 parts of potassium sorbate and 8-10 parts of talcum powder. The softener for bamboo weaving has a good softening effect, is non-corrosive and convenient to popularize and use and has a better effect on improvement of strength and toughness of a softened part.
Owner:陈新棠
Production method of phenolic foam without formaldehyde release
InactiveCN102443244ALow free formaldehyde contentSimple operation processThermal insulationPhosphoric acid
The invention relates to a production method of phenolic foam without formaldehyde release, which adopts the following raw materials and the steps of: (1) preparing for phenol, industrial paraformaldehyde, sodium hydroxide, hydrogen peroxide, methyl acetoacetate and water; (2) adding the phenol, the sodium hydroxide and the water into a reaction kettle, heating up and adding the industrial paraformaldehyde; (3) performing vacuum dehydration; (4) adding the hydrogen peroxide and the methyl acetoacetate, and stirring; (5) taking thermosetting phenolic resin obtained in the step (4), H-330 organic silicon and petroleum ether and stirring; and (6) adding p-toluenesulfonic acid, phosphoric acid and the water, stirring and foaming to obtain the phenolic foam. According to the production method disclosed by the invention, acid is not required to be added for regulating PH value, so that operation process is simplified and economic benefits are significant. Furthermore, compared with the traditional phenolic foam, the phenolic foam has the advantages of obviously reducing the content of free formaldehyde, and avoiding the formaldehyde release during the use, and can be widely applied to the fields including air pipes of central air conditioners, heat insulation of interior walls, heat insulation of clean rooms and other places with high requirements on environmental protection.
Owner:湖南中野高科技特种材料有限公司
Process of continuously producing methyl acetoacetate
InactiveCN105384631AFast outputIncrease finished productOrganic compound preparationPreparation from ketenes/polyketenesAcetic acidNitrogen
The invention discloses a process of continuously producing methyl acetoacetate and relates to the technical field of production of chemical raw materials. The production process comprises the following steps of firstly, welding a separation board at the middle of an esterification reactor for separating the esterification reactor into a cooling bin and an insulating bin; secondly, adding a methyl acetoacetate finished product into the esterification reactor to serve as a bottom material in the reactor, and meanwhile, simultaneously dropping DK and methanol into the cooling bin; thirdly, realizing water cooling and air cooling in the cooling bin; after the cooling bin is cooled, realizing pressurization; after pressurization, enabling a product to overflow to the inside of the insulating bin through an overflow pipe; during overflowing, adding diketene on the overflow pipe; fourthly, when the product in the insulation bin reaches a certain amount, transferring the methyl acetoacetate to the inside of a rectifying tower through a transfer pump; fifthly, vacuumizing the inside of the rectifying tower for exhausting air, and meanwhile, inputting nitrogen, and rectifying the methyl acetoacetate in a reflux state to obtain a final product at the moment. According to the process disclosed by the invention, quick production can be realized, the finished product output is improved, the accuracy in control is realized, and the time and the cost are saved.
Owner:JIANGSU TIANCHENG BIOCHEM PROD
Method for preparing methyl acetoacetate
InactiveCN103864616AHigh purityRaise the ratioPreparation from ketenes/polyketenesCarboxylic acid esters separation/purificationRefluxAcetic acid
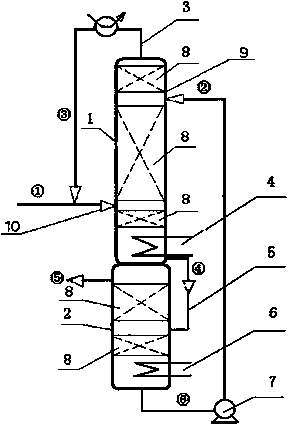
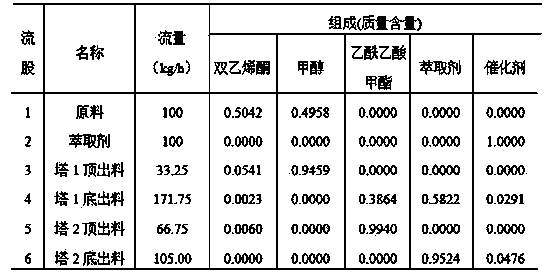
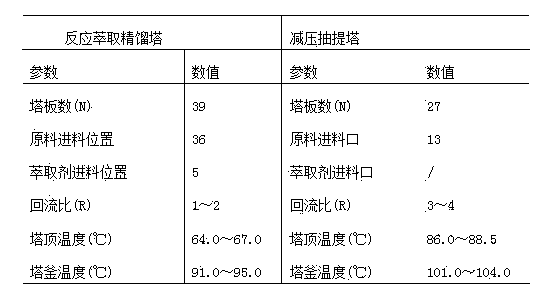
The invention discloses a method for preparing methyl acetoacetate. The method comprises the following steps: adding a catalyst once, continuously adding ketene dimer and a methanol solution through a raw material feeding hole, continuously adding an extraction agent through an extraction agent feeding hole, simultaneously adding two materials into a reaction-extraction rectifying tower, and starting heating; carrying out total reflux so as to obtain the methanol solution through the tower top of the reaction-extraction rectifying tower and obtain a mixed liquid of the methyl acetoacetate, the catalyst and the extraction agent through the tower bottom of the reaction-extraction rectifying tower, and enabling the mixed liquid to flow into a decompression extraction tower from a tower-bottom discharging hole, so as to obtain a target product, namely the methyl acetoacetate from the tower top of the decompression extraction tower. Due to the arrangement of the reaction-extraction rectifying tower and the decompression extraction tower, the methyl acetoacetate is prepared by virtue of a reaction-rectification and extraction-separation integrated reaction, the materials are fed once, and the catalyst and the extraction agent can be recycled, so that the operation is simple, the cost is lowered, the purity and rate of the prepared methyl acetoacetate are high, and the energy consumption is reduced.
Owner:NANJING NORMAL UNIVERSITY
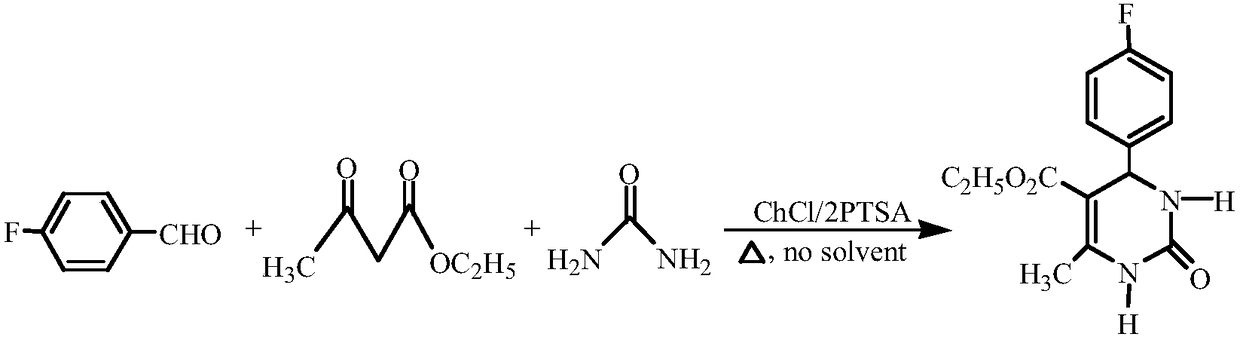
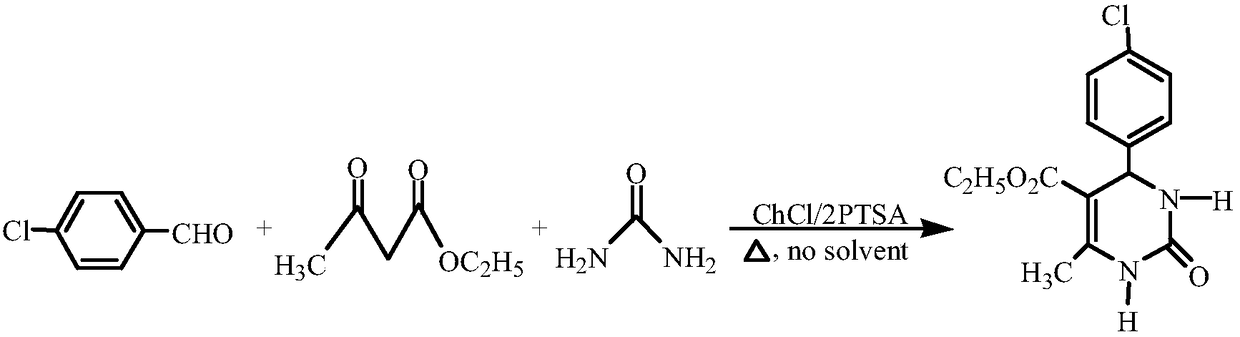
Method for synthesizing 4-halophenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydro-pyrimidin-2(1H)-one
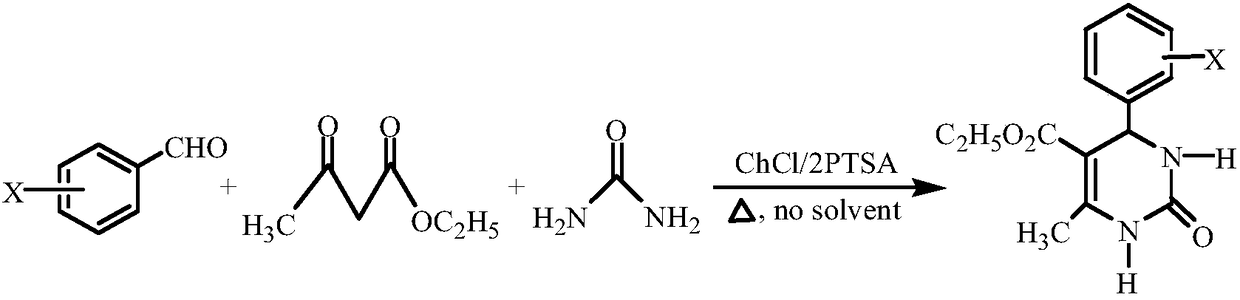
The invention relates to a method for synthesizing 4-halophenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydro-pyrimidin-2(1H)-one. The method specifically comprises the following steps: with halogenated benzaldehyde, ethyl acetoacetate and urea as a substrate, and DES as a catalyst, stirring for reacting at a temperature of 60-75 DEG C under a solvent-free condition for 30-45 minutes, wherein a molar ratio of aromatic aldehyde to methyl acetoacetate to urea to DES is 1:1:1.5:0.3. According to the method disclosed by the invention, the cheap and readily available DES serves as the catalyst, any other solvent does not need to be added in the reaction process, ny other solvent and corrosive catalyst used can be effectively decreased, the reaction conditions are mild, and the method can be repeatedlyutilized and is simple in process, high in catalytic activity and high in yield; and moreover, the method is simple and convenient in reaction after-treatment, has environmental-friendly effect and isa cheap, safe and environmental-friendly synthetic method.
Owner:DALIAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com