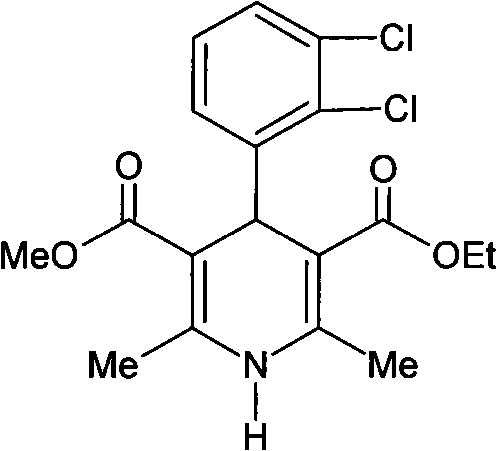
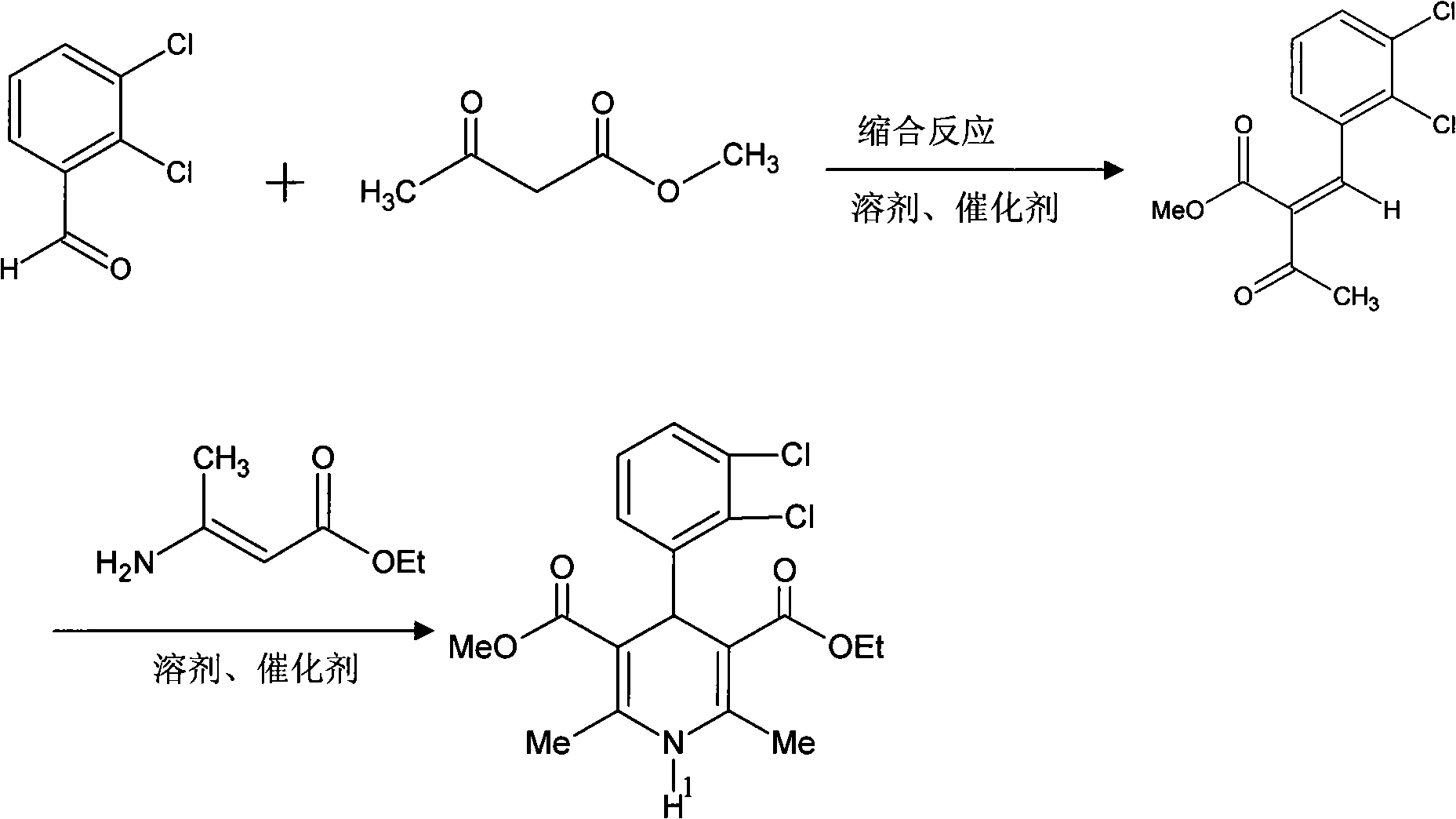
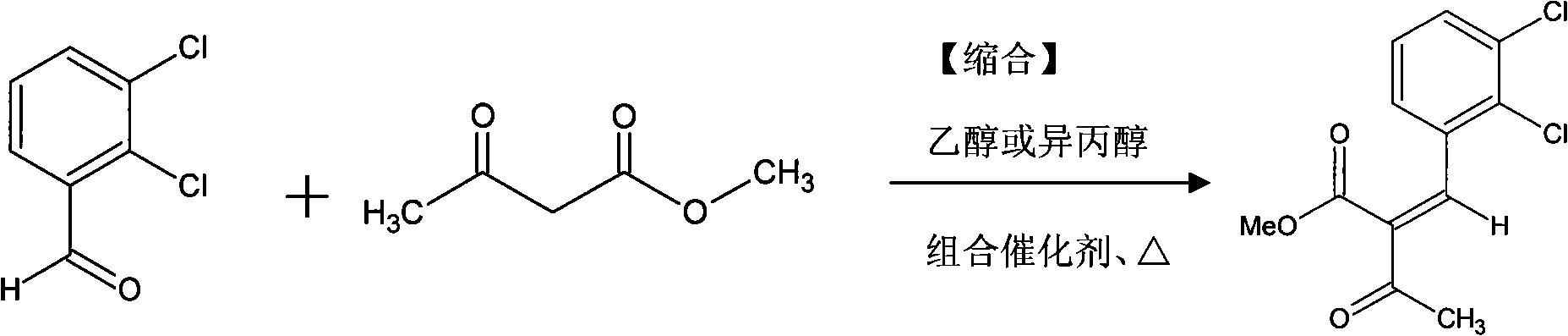
Preparation method of felodipine synthetic intermediate methyl 2-(2,3-dichlorobenzylidine)acetoacetate
A technology of methyl dichlorobenzylidene acetoacetate and methyl acetoacetate, which is applied in the field of organic chemical synthesis to achieve the effects of clean production, good crystal form, and bright color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Example 1: Preparation of felodipine synthetic intermediate-2,3-dichlorobenzylidene acetoacetate methyl ester
[0030] Put 600ml of absolute ethanol, 119.2g (pure: 1.02mol.) methyl acetoacetate, 3.53g (pure: 41mmol.) piperidine, 7.68g (pure: 44mmol.) into a 1000ml. 2-quinolinecarboxylic acid and 141.4 g (reduced purity: 0.8 mol) of 2,3-dichlorobenzaldehyde. After the addition was completed, the temperature of the water bath was raised to 41° C. to 45° C., and the stirring was continued for 6 hours (the end point of the reaction was determined by TLC). Place the reaction solution in a cold water bath at 15°C±2°C and stir for crystallization for 2 hours, filter the mother liquor, wash the filter cake with an appropriate amount of cold solvent, and dry it in vacuum below 50°C to obtain a white shiny intermediate crystal (one time precipitates). Yield 138.6 g., purity 98.7%.
[0031] Combine the mother liquor and washing liquid of the primary precipitate, concentrate in ...
example 2
[0032] Example 2. Felodipine synthetic intermediate-2, the preparation of 3-dichlorobenzylidene acetoacetate methyl ester
[0033] In this embodiment, isopropanol (640ml.) is used instead of absolute ethanol as the condensation reaction and recrystallization solvent, and all other raw materials, catalyst specifications, dosages and ratios are the same as in Example 1, and are obtained according to the preparation method of Example 1 once Precipitate 140.1g., purity 98.5%; secondary precipitate crude product 50.3g., purity 95.6%. The crude product was purified once with isopropanol to obtain 44.3 g of the secondary product with a purity of 99.1%. Condensation and refining total yield: 83.06%.
example 3
[0034] Example 3. Felodipine synthetic intermediate-2, the preparation method of 3-dichlorobenzylidene acetoacetate methyl ester (the comparative patent method of example 1)
[0035] Dissolve 141.4g (purity 0.8moL) of 2,3-dichlorobenzaldehyde in 660ml.isopropanol, then add 4.97g (purity 0.04mol) of picolinic acid, 3.44g (purity 0.04mol) ) piperidine and 119.6 g (1.02 mol of pure) of methyl acetoacetate. After the addition is complete, the temperature of the water bath is raised to 40-45° C., and the reaction is stirred for 6 hours. The reaction solution was stirred and crystallized on a water bath at 15°C±2°C for 2 hours. The mother liquor was filtered off in vacuo, and the filter cake was washed with a small amount of cold isopropanol and then vacuum-dried to dryness below 50° C. to obtain 131.4 g of off-white powder crystals with a purity of 97.6% (HPLC normalization method).
[0036] The mother liquor of the primary precipitate was combined with the washing liquid, concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com