Method for preparing butyrate clevidipine
A technology of clevidipine butyrate and formic acid, which is applied in the direction of organic chemistry, can solve the problems of many impurities, not suitable for mass production of products, and difficult control of synthesis reactions, etc., and achieve the effect of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
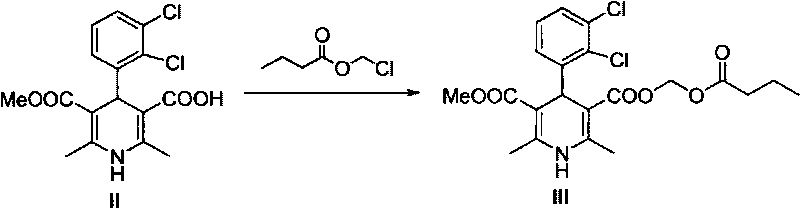
[0026] Embodiment 1: (±)-4-(2', 3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-5-carboxylic acid methyl-3-formic acid ( II)
[0027] In a round bottom flask, add 2,3-dichlorobenzaldehyde (175g, 1mol), propionitrile acetoacetate (160g, 1.03mol) and 3-amino-2-butenoic acid methyl ester (115g, 1mol), iso Propanol was used as a solvent, and the reaction was carried out at 50°C for 12 hours. After removing the solvent under reduced pressure, cool to room temperature, add ethanol / water mixed solution of sodium hydroxide, stir for 5 h, then add 1 L of water, and wash the aqueous phase with dichloromethane. 300 mL of phosphoric acid was slowly added to the water phase, and a yellow precipitate was formed, and 273.2 g of solid was obtained by suction filtration, with a yield of 77%. IR (KBr, cm -1 ): 3344, 2950, 1656, 1477, 1229, 779. 1 H NMR (DMSO, ppm): δ=11.59(s, 1H), 8.81(s, 1H), 7.38-7.21(m, 3H), 5.30(s, 1H), 3.48(s, 3H), 2.23(s , 3H), 2.22(s, 3H); 13 C NMR (DMSO, ppm...
Embodiment 2
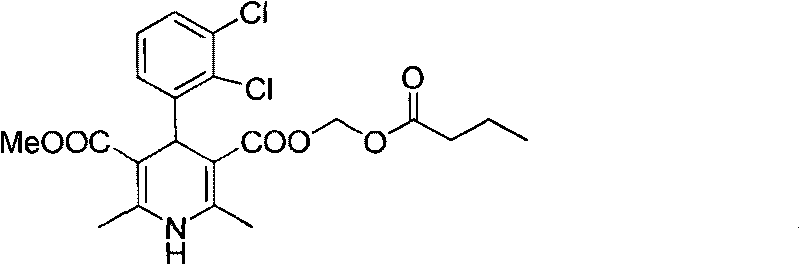
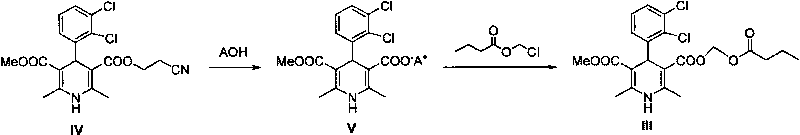
[0028] Example 2: (±)-4-(2', 3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid methyl (butyl Acyloxymethyl) ester (III)
[0029] Add (±)-4-(2',3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-5-carboxylic acid methyl-3- Formic acid (171.0g, 0.48mol), anhydrous sodium carbonate (101.8g, 0.96mol) and 3L of DMF were added, and chloromethyl n-butyrate (61.8mL, 0.49mol) was added, and the reaction was stirred at 60°C for 12h. The reaction liquid was cooled to room temperature, 2L of ethyl acetate and 1L of water were added, the organic phase was separated, and the solvent was removed after drying to obtain a yellow solid. Repeated recrystallization with ethyl acetate / petroleum ether gave 97.5 g of white (slightly bright yellow) solid, with a yield of about 45%. Mp.136.4°C-138.9°C. Optical rotation: [ a ] D 20 = 0 (c=5, MeOH). The purity by HPL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com