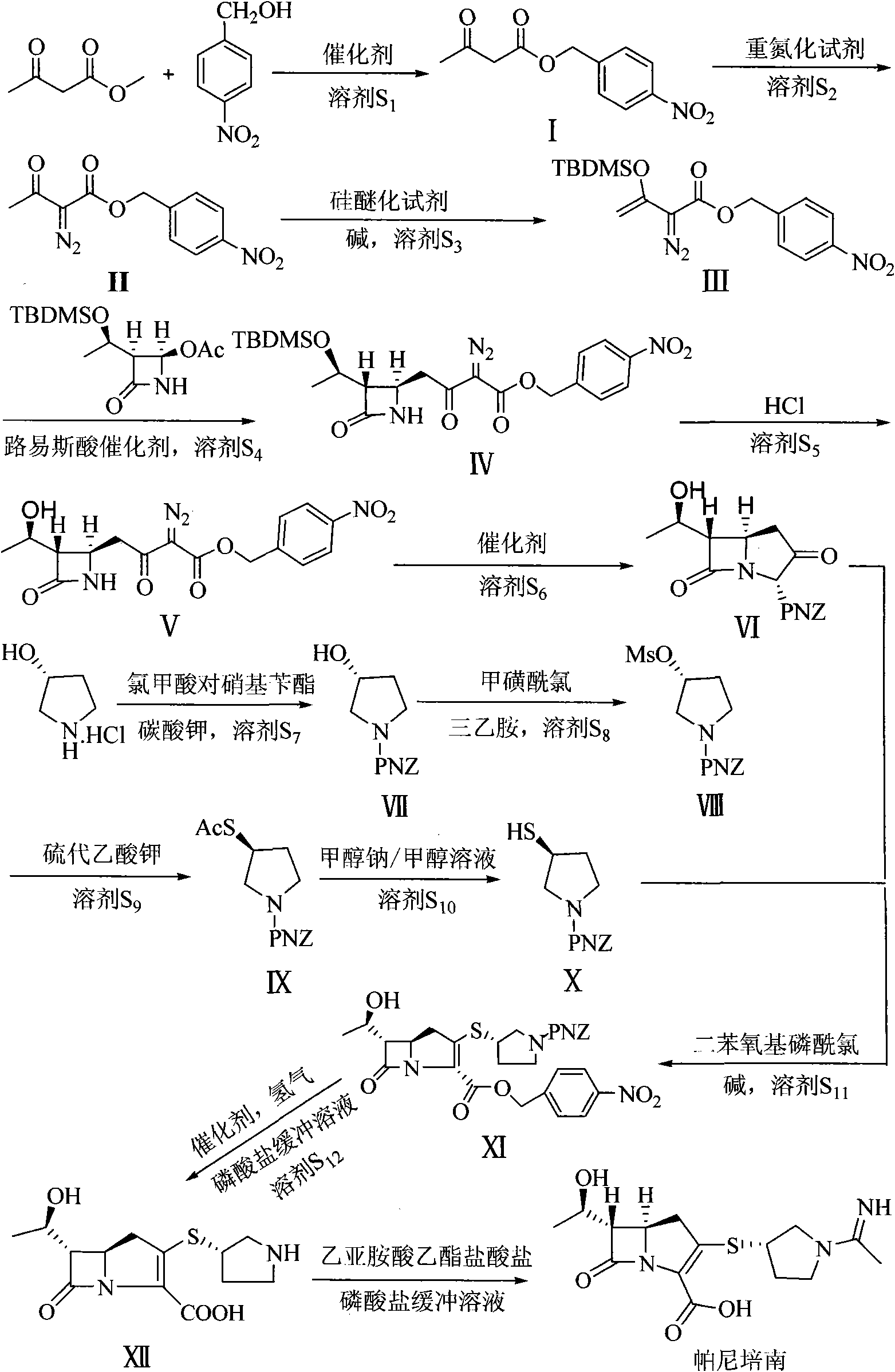
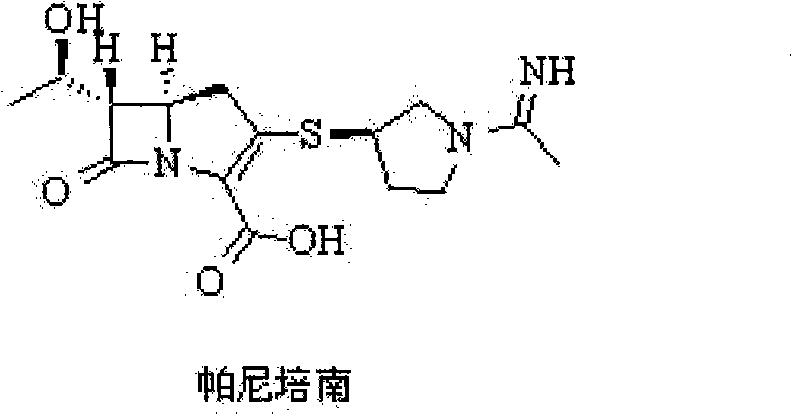
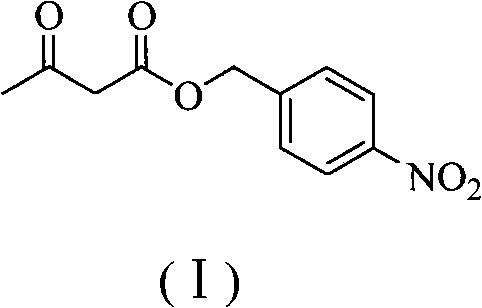
Preparation method of panipenem
A solvent and p-nitrobenzyl ester technology, which is applied in the field of preparation of panipenem, can solve the problems of many imidization side reactions, harsh reaction conditions for the synthesis of panipenem, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Add 5.8g (50mmol) methyl acetoacetate, 7.65g (50mmol) p-nitrobenzyl alcohol and 0.31g (5mmol) to a 250mL round bottom flask, B(OH) 3 Then add 100mL of anhydrous toluene and heat to reflux at 110°C for 5h. After the reaction was complete, the solvent was removed under reduced pressure, and the crude product was purified by silica gel column chromatography to obtain 9.95 g of light yellow solid: p-nitrobenzyl acetoacetate (I), with a yield of 84%, m.p.: 42-44°C.
[0061] The nuclear magnetic spectrum of p-nitrobenzyl acetoacetate is: 1 H NMR (400MHz, CDCl 3 ): δ2.30(s, 3H), 3.60(s, 2H), 5.29(s, 2H), 7.55(d, J=8.0Hz, 2H), 8.22(d, J=8.0Hz, 2H); 13 CNMR (100MHz, CDCl 3 ): δ30.3, 49.8, 65.4, 123.7, 128.4, 142.6, 147.8, 166.6, 200.1.
[0062] Elemental analysis is: C 11 h 11 NO 5 Theoretical values for: C, 55.70; H, 4.67; N, 5.90. Found: C, 55.72; H, 4.65; N, 5.93.
[0063] (2) 2.37g (10mmol) p-nitrobenzyl acetoacetate (I) and 40mL acetonitrile were added to a 1...
Embodiment 2
[0101] Adopt DMAP to make catalyst in the step (1), the mol ratio of p-nitrobenzyl alcohol and methyl acetoacetate is 1: 5, 60 ℃ of reaction temperature, the reaction time is 30h, all the other are identical with embodiment 1, productive rate: 80%
[0102] In the step (2), the diazotization reagent is p-toluenesulfonyl azide, the mol ratio of p-nitrobenzyl acetoacetate to p-toluenesulfonyl azide is 1:8, the solvent is tetrahydrofuran, and the reaction temperature is -15°C. The time is 30h, the rest are the same as in Example 1, and the yield is 88%.
[0103] In the step (3), the silicon etherification reagent is trimethylchlorosilane, the base is diethylamine, and the mol ratio of diazotized acetoacetate to p-nitrobenzyl, trimethylchlorosilane and diethylamine is 1: 5:3, the reaction temperature is -20°C, the reaction time is 15h, the rest is the same as in Example 1, and the yield is 94%.
[0104] Lewis acid catalyst is ZnBr in step (4) 2 , 4-acetoxy-3-[1-(tert-butyldimethy...
Embodiment 3
[0116] In the step (1), NBS is used as a catalyst, tetrahydrofuran is a solvent, the mol ratio of p-nitrobenzyl alcohol to methyl acetoacetate is 1: 10, the reaction temperature is 85°C, and the reaction time is 17h. The rest are the same as in Example 1, and the product Rate: 75%.
[0117] In step (2), the molar ratio of p-nitrobenzyl acetoacetate to p-toluenesulfonyl azide is 1:4, the reaction temperature is 20° C., and the reaction time is 15 h. The rest are the same as in Example 1, and the yield is 90%.
[0118] In step (3), the mol ratio of p-nitrobenzyl diazotized acetoacetate, tert-butyldimethylsilyl trifluorosulfonate and triethylamine is 1:3:1, the solvent is tetrahydrofuran, and the reaction temperature 25°C, the reaction time is 9h, the rest is the same as Example 1, and the yield: 92%.
[0119] Lewis acid catalyst is ZnI in step (4) 2 , 4-acetoxy-3-[1-(tert-butyldimethylsilyloxy)ethyl]azetidinone, 2-diazo-3-tert-butyldimethylsilyloxy-3 - p-nitrobenzyl crotonate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com