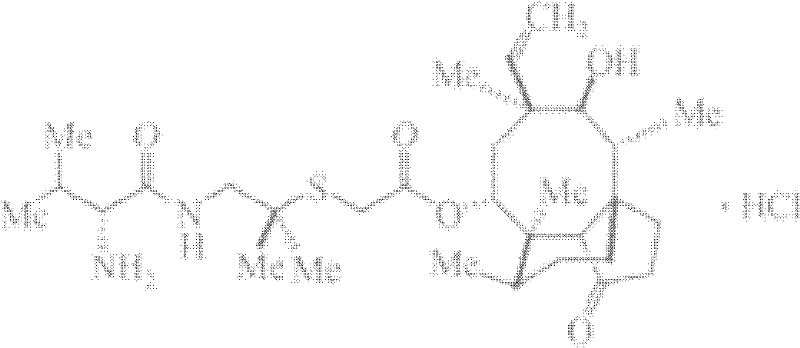
Preparation method of valnemulin hydrochloride
A technology of vornimulin hydrochloride and acetic acid, which is applied in the field of preparation of vornimulin hydrochloride, can solve the problems of easily polluting the environment, difficult regeneration of palladium catalysts, etc., and achieves the effects of reducing environmental pollution, less solvent, and easy mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
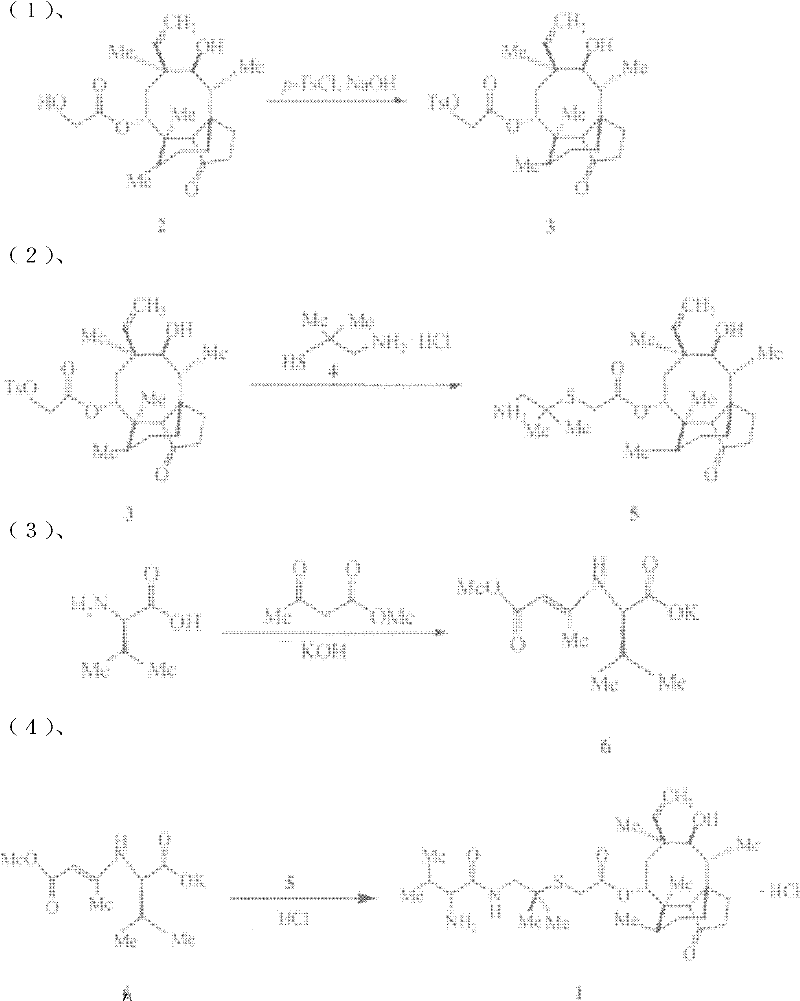
Examples
Embodiment 1
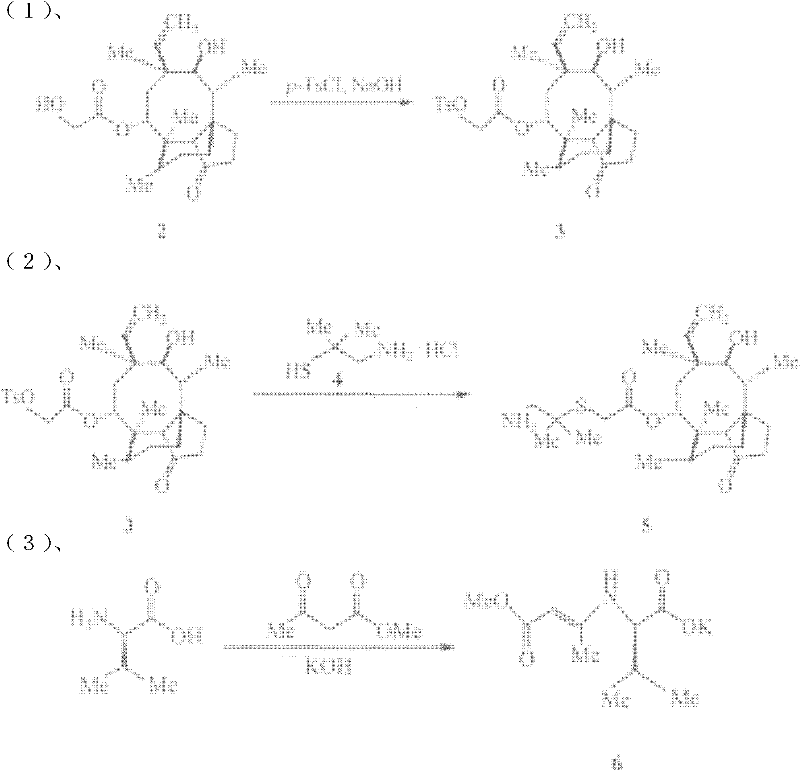
[0015] Preparation of 2-(4-methylbenzenesulfonyloxy)acetic acid (3aS, 4R, 5S, 6S, 8R, 9R, 9aR, 10R)-6-vinyldecahydro-5-hydroxy-4,6,9, 10-Tetramethyl-1-oxo-3a,9-propanol-3aH-cyclopentacycloocten-8-yl ester (substance 3 in the scheme).
[0016] Dissolve 75.7 g of pleuromutilin in methanol (200 ml). Water (40ml) was added, cooled in an ice-water bath to 0°C and stirred for 15min. A mixture of 42.0 g of p-toluenesulfonyl chloride and water (120 ml) was added dropwise. After dropping, stir at 100°C for 1000min. Cool to 0°C in an ice-salt bath, slowly drop into 10mol / L sodium hydroxide aqueous solution (50ml), heat to reflux for 0.5h and then cool to room temperature. Cool in an ice-salt bath to 0°C, add water (40ml), filter, wash the filter cake with water (20ml×3) and cold methanol (20ml×3) in sequence, and dry at 100°C to obtain a white solid (substance 3 in the reaction formula) .
[0017] Preparation of [(2-amino-1,1-dimethylethyl)thio]acetic acid (3aS, 4R, 5S, 6S, 8R, 9R,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com