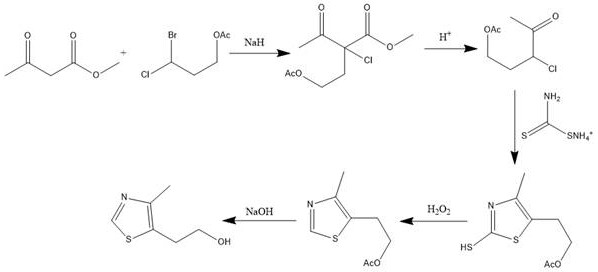
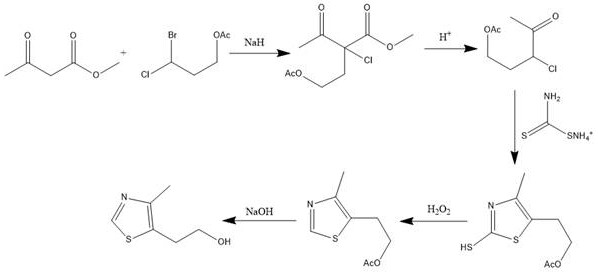
Synthetic method of 5-(2-hydroxyethyl)-4-methylthiazole
A synthesis method and thiothiazole technology, applied in the direction of organic chemistry and the like, can solve the problems of long steps, many side reactions, low yields, etc., and achieve the effects of mild reaction conditions, saving reaction time, and simple post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 11.6g (0.1mol) of methyl acetoacetate in 300ml of anhydrous tetrahydrofuran, at 0°C, under the protection of nitrogen, add 4.8g (0.12mol) of 60% sodium hydrogen in batches, and then drop in 21.5g (0.1mol) ) 50ml tetrahydrofuran solution of 3-bromo-3-chloropropyl acetate, after putting in, continue to react for 2 hours, then heat up to reflux for 0.5-1 hour. After the reaction was detected by GC-MS, the temperature was lowered to 0°C, and a sufficient amount of 5% dilute hydrochloric acid was slowly added dropwise to adjust the pH to neutral, extracted with ether, and then the ether was distilled off to obtain 24 g of 4-acetoxy- The crude product of methyl 2-acetyl-2-chlorobutyrate was 95% of methyl 4-acetoxy-2-acetyl-2-chlorobutyrate by GC-MS, and 2.8% of 4- Acetoxy-2-acetyl-2-bromobutyric acid methyl ester, the content of disubstituted impurities is less than 1%, and the content of non-halogenated impurities is less than 1%. No further purification is needed, ...
Embodiment 2
[0032] Dissolve 200g (1.72mol) of methyl acetoacetate in 1.5L of anhydrous tetrahydrofuran, and at -5°C, under nitrogen protection, add 72g (1.80mol) of 60% sodium hydrogen in batches, and then drop 369.8g (1.72mol) ) 3-bromo-3-chloropropyl acetate, input is completed, continue to react for 2 hours, then heat up to reflux for 1 hour. After the reaction was detected by GC-MS, the temperature was lowered to 0°C, and a sufficient amount of 5% dilute hydrochloric acid was slowly added dropwise to adjust the pH to neutral, extracted with ether, and then the ether was distilled off to obtain 240 g of 4-acetoxy- 2-acetyl-2-chlorobutyric acid methyl ester crude product, 408g of 4-acetoxy-2-acetyl-2-chlorobutyric acid methyl ester crude product with 96% content detected by GC-MS, no further purification needed, directly into the next reaction.
[0033] Add 408 g of the crude product 4-acetoxy-2-acetyl-2-chlorobutyric acid methyl ester prepared above into a 2L reaction flask, add 1.2L ...
Embodiment 3
[0038]Put 5kg of methyl acetoacetate into a 50L reaction kettle, add 20L of anhydrous tetrahydrofuran to dissolve it, and put 1.81kg of 60% sodium hydrogen in batches at -10~-5°C under the protection of nitrogen, and then drop in 8.62kg of 3- Bromo-3-chloropropyl acetate, the input was completed, and the reaction was continued for 8 hours, and then the temperature was raised to room temperature for 2 hours. After the reaction was detected by GC-MS, lower the temperature to -5°C, slowly drop in a sufficient amount of 10% dilute hydrochloric acid, adjust the pH to neutral, extract with ether, and then distill off the ether to obtain 9.98kg of 4-acetoxy The crude product of methyl-2-acetyl-2-chlorobutyrate was 97% 4-acetoxy-2-acetyl-2-chlorobutyric acid methyl ester by GC-MS, without purification, directly Enter the next reaction.
[0039] Add 9.98kg of the crude product 4-acetoxy-2-acetyl-2-chlorobutyrate methyl ester prepared above in a 100L reactor, add 50L of 20% sulfuric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com