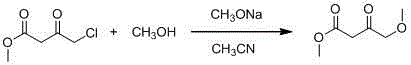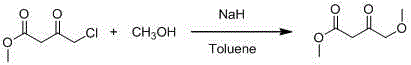4-methoxy methyl acetoacetate preparation method
A technology of methyl methoxyacetoacetate and methyl acetate, which is applied in the field of preparation of methyl 4-methoxyacetoacetate, can solve problems such as unfavorable industrialized large-scale production, achieve safe use, reduce impurity content, reduce The effect of the introduction of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 2L reaction flask, add 250ml of solvent tetrahydrofuran (AR) in advance, and start stirring under the protection of argon. At this time, the temperature in the kettle is 25°C, and add 30g (0.75mol) of industrial sodium hydride (containing 40wt% mineral oil) in batches. And 53g (0.75mol) of potassium methylate, after the addition, continue to add 450ml of solvent tetrahydrofuran; slowly drop the mixed solution of 30g of methanol and 100g (0.68mol) of methyl 4-chloroacetoacetate at the temperature of 20°C in the kettle, and stir for 4h , raise the temperature in the kettle to 25°C and stir for 5 hours. TLC detects that the reaction is complete, and the system begins to cool down. The color of the solution is earthy yellow and a large amount of solids are suspended. to 0-5°C) until the pH of the system = 5; stand for stratification, after separation, the upper layer is concentrated and spin-dried to remove the solvent tetrahydrofuran, and the resulting residue is added...
Embodiment 2
[0021] In a 2L reaction flask, add 250ml of solvent tetrahydrofuran (AR) in advance, and start stirring under the protection of argon. At this time, the temperature in the kettle is 15°C, and add 30g (0.75mol) of industrial sodium hydride (containing 40wt% mineral oil) in batches. and 40.5g (0.75mol) sodium methoxide, after the addition, continue to add 450ml of solvent tetrahydrofuran; slowly add a mixed solution of 30g methanol and 100g (0.68mol) methyl 4-chloroacetoacetate dropwise at a temperature of 15°C in the kettle, and stir the reaction 6h, raise the temperature in the kettle to 20°C and stir for 15h, TLC detects that the reaction is complete, the system begins to cool down, the color of the solution is earthy yellow and a large amount of solids are suspended, when the temperature in the kettle drops to 10°C, slowly add 2M hydrochloric acid solution (pre- Cool to 0-5°C) until the pH of the system is 7; stand and separate the layers, concentrate the upper layer and spin...
Embodiment 3
[0023] In a 2L reaction flask, add 250ml of solvent tetrahydrofuran (AR) in advance, and start stirring under the protection of argon. At this time, the temperature in the kettle is 20°C, and add 30g (0.75mol) of industrial sodium hydride (containing 40wt% mineral oil) in batches. and 51g (0.75mol) sodium ethoxide, after the addition, continue to add 450ml of solvent tetrahydrofuran; slowly add dropwise a mixed solution of 30g methanol and 100g (0.68mol) methyl 4-chloroacetoacetate at a temperature of 5°C in the kettle, and stir for 5h , raise the temperature in the kettle to 25°C and stir for 4 hours. TLC detects that the reaction is complete, and the system begins to cool down. The color of the solution is earthy yellow and a large amount of solids are suspended. to 0-5°C) until the pH of the system = 6; stand for stratification, and after separation, the upper layer is concentrated and spin-dried to remove the solvent tetrahydrofuran, and the resulting residue is added with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com