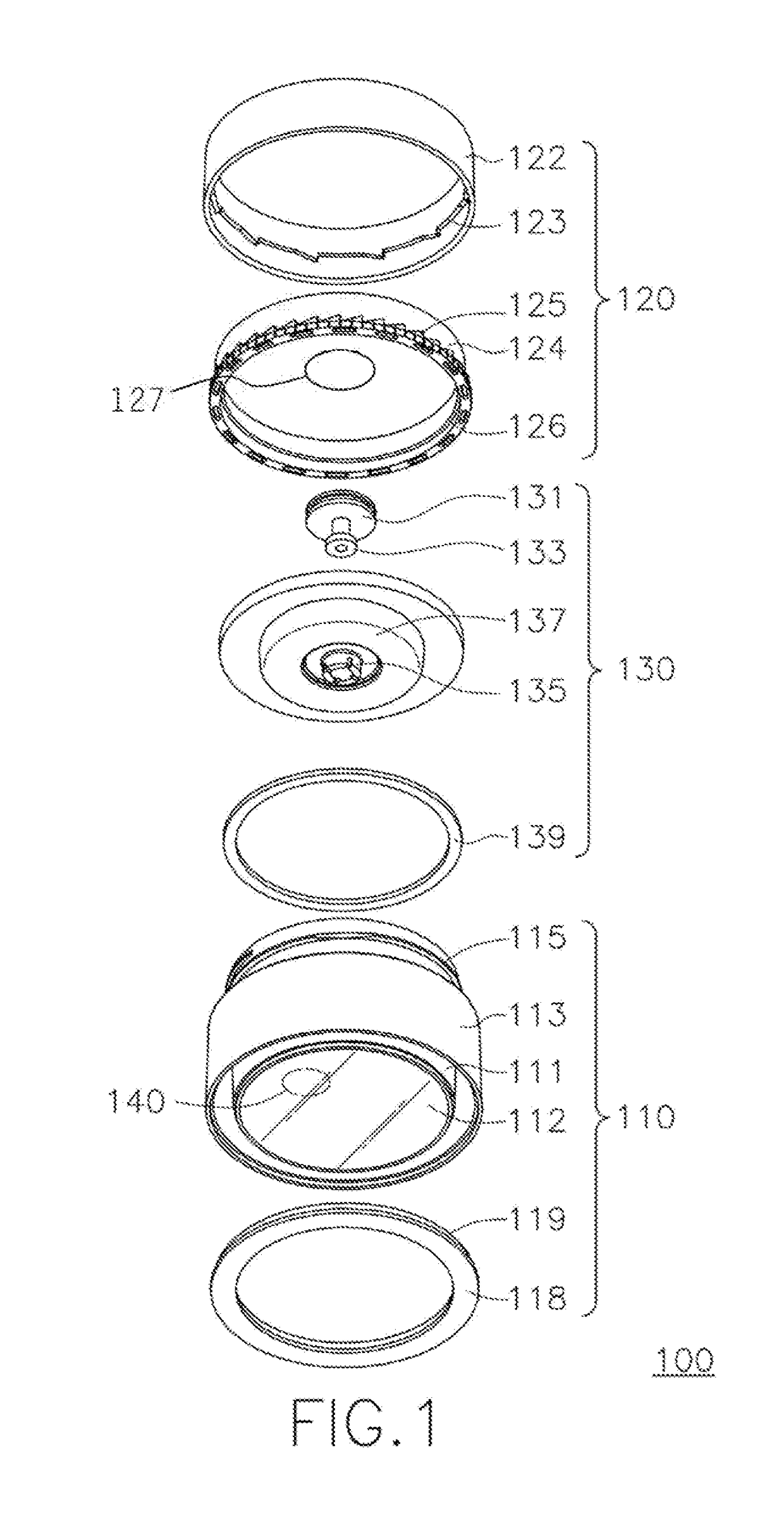
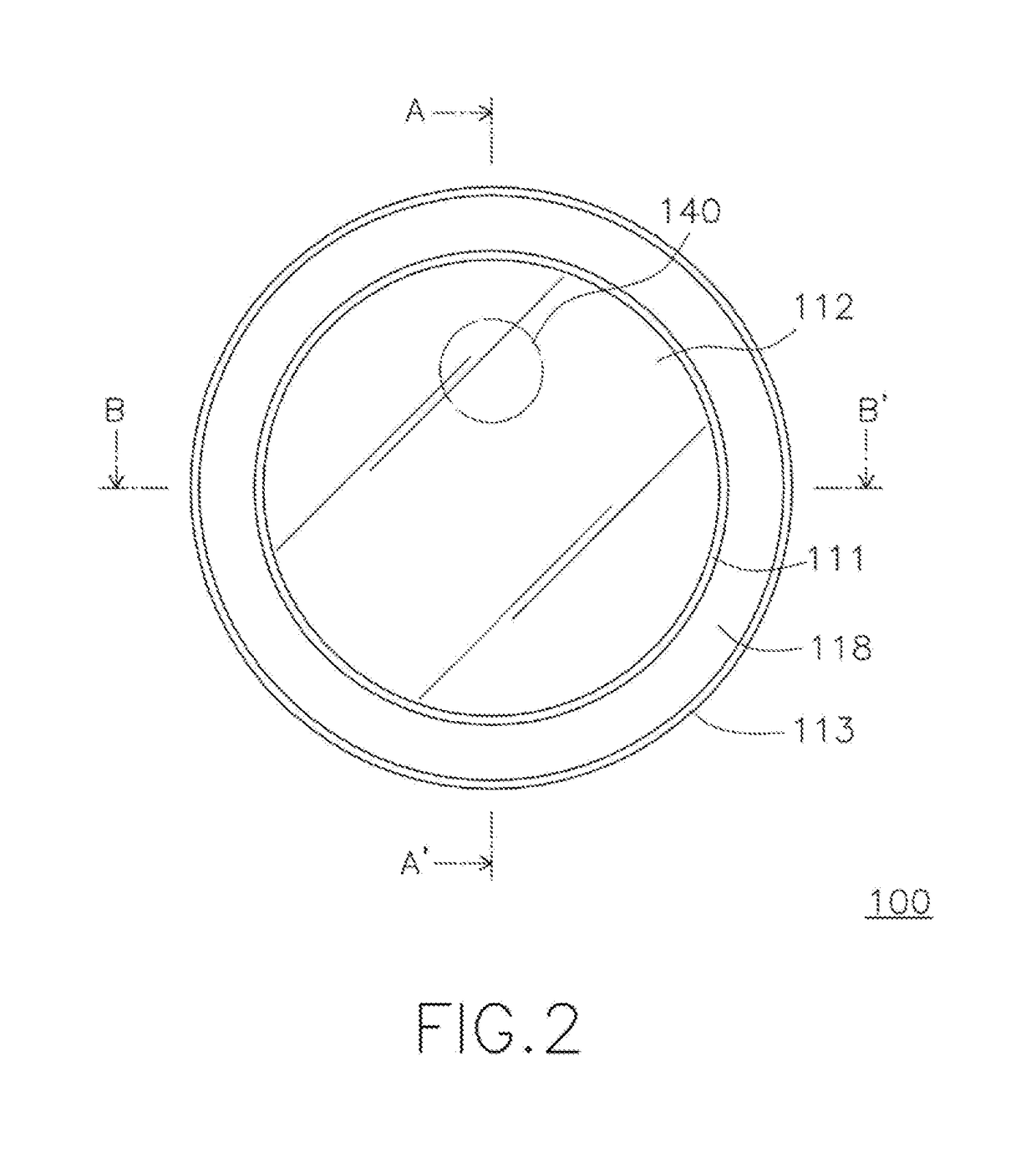
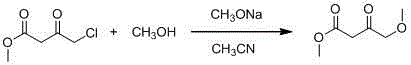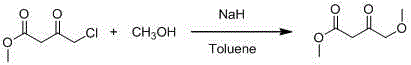Patents
Literature
178results about How to "Good save" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
File based database synchronization method
InactiveCN102752372AGood saveImprove synchronization efficiencyTransmissionSpecial data processing applicationsData synchronizationData library
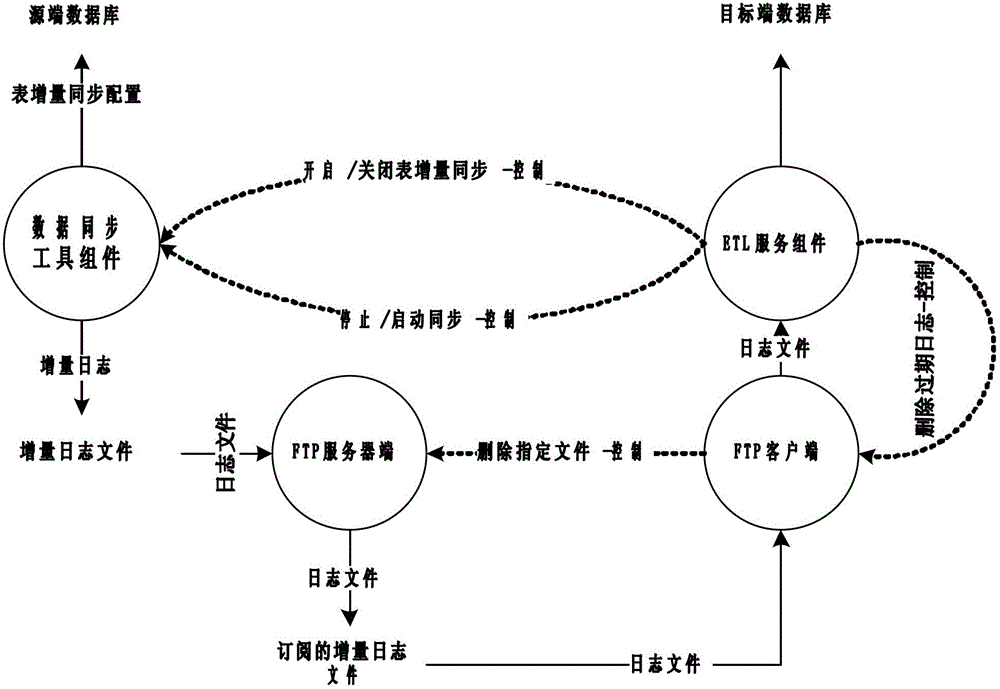
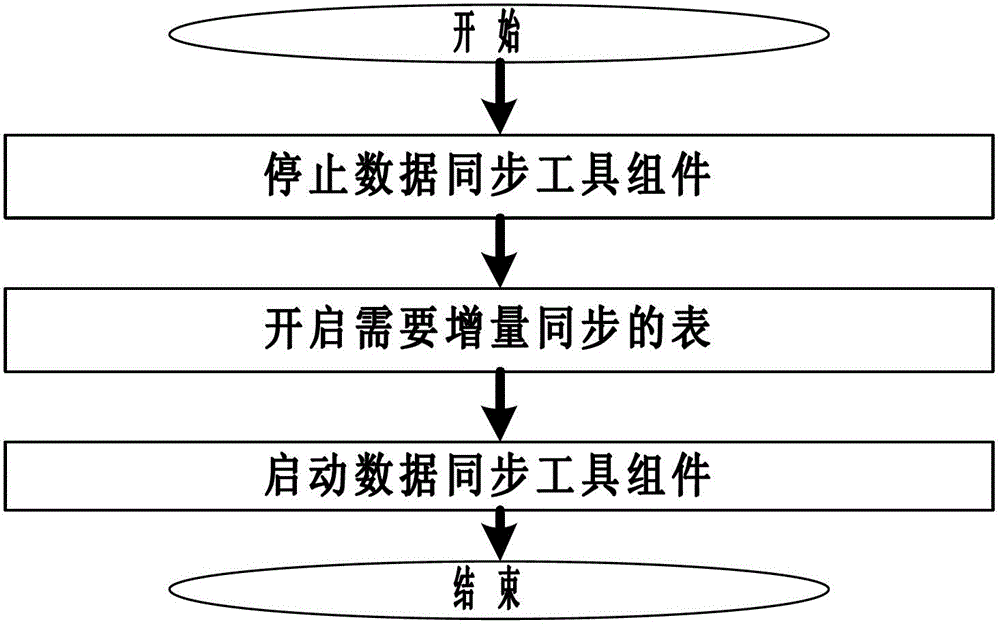
The invention relates to a file based database synchronization method, which has the following main technical features that through a mode of installing a data synchronization tool component in a source-end database and installing an ETL (extract transform and load) service component in a target-end database, the control between the ETL service component and the data synchronization tool component is implemented through a protocol, and the synchronization is implemented from the source-end database and the target-end database in a file mode. The method disclosed by the invention is reasonable in design; because the database synchronization is implemented from the source-end database and the target-end database in a file mode, the database data required to be synchronized can be efficiently stored, and in a heterogeneous database and an operating system environment, the synchronous operation of data can be implemented through file analysis, thereby supporting a characteristic of carrying out synchronization on various heterogeneous databases, meanwhile, by using a total synchronization method and an incremental synchronization method, the efficiency of database synchronization is improved.
Owner:天津神舟通用数据技术有限公司
Production Method of Nitrogen-Containing Fused Ring Compounds
InactiveUS20080064871A1Good chemical stabilityImprove physical stabilityOrganic chemistryMetabolism disorderPurification methodsCoronary artery disease
[Problems] The present invention provides a superior production method and a superior purification method of compounds effective for the treatment or prophylaxis of pathology showing involvement of uric acid, such as hyperuricemia, gouty tophus, acute gouty arthritis, chronic gouty arthritis, gouty kidney, urolithiasis, renal function disorder, coronary artery disease, ischemic heart disease and the like. [Means] A compound represented by the following formula [2] or a pharmaceutically acceptable salt thereof can be produced by reacting a compound represented by the following formula [3] or a salt thereof with a compound represented by the following formula [4], a salt thereof or a reactive derivative thereof. Moreover, crystallization of a compound represented by the formula [2] can be performed with industrially superior workability, and high quality crystals of a compound represented by the formula [2] can be obtained. wherein each symbol is as defined in the description.
Owner:JAPAN TOBACCO INC
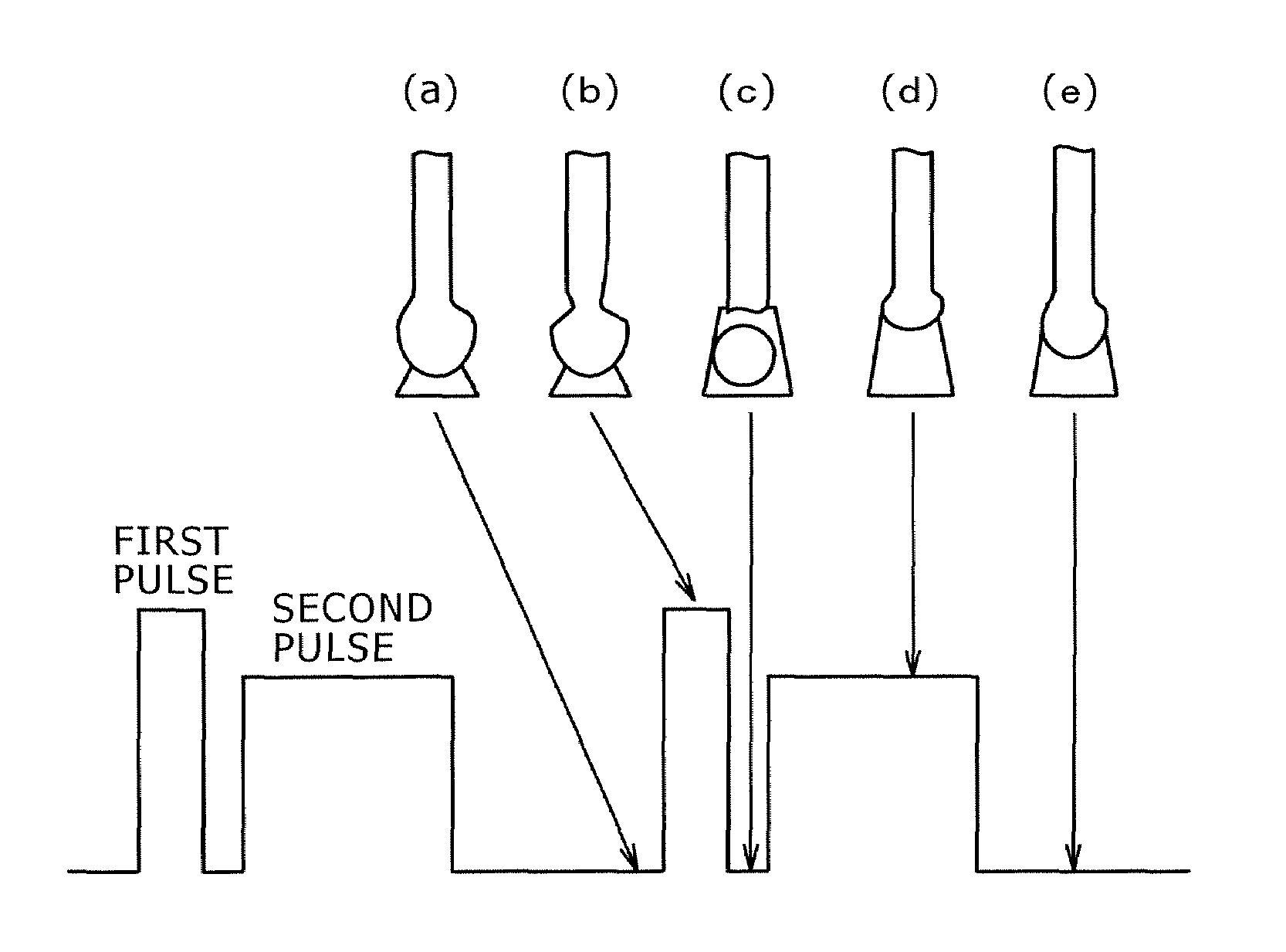
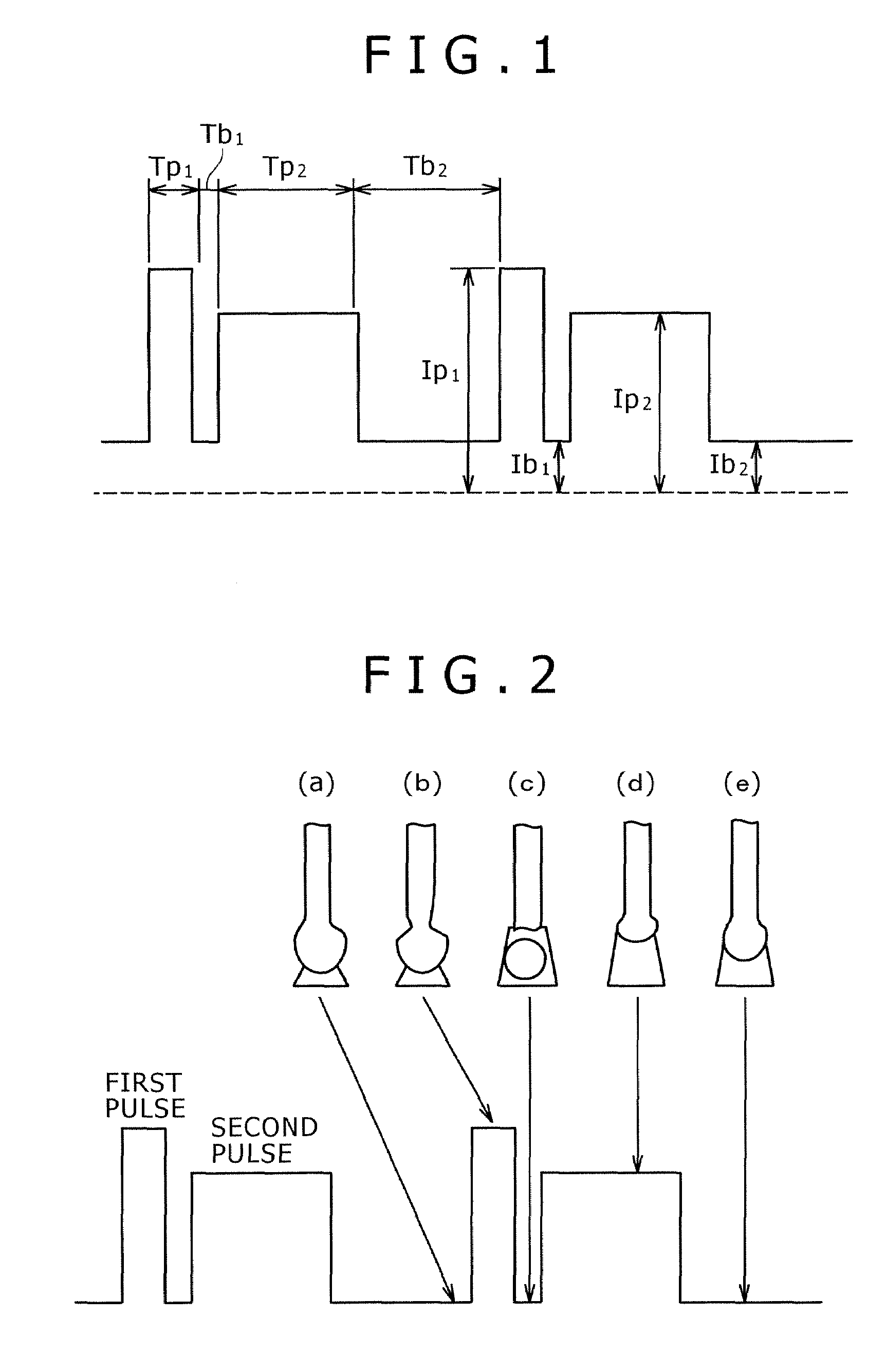
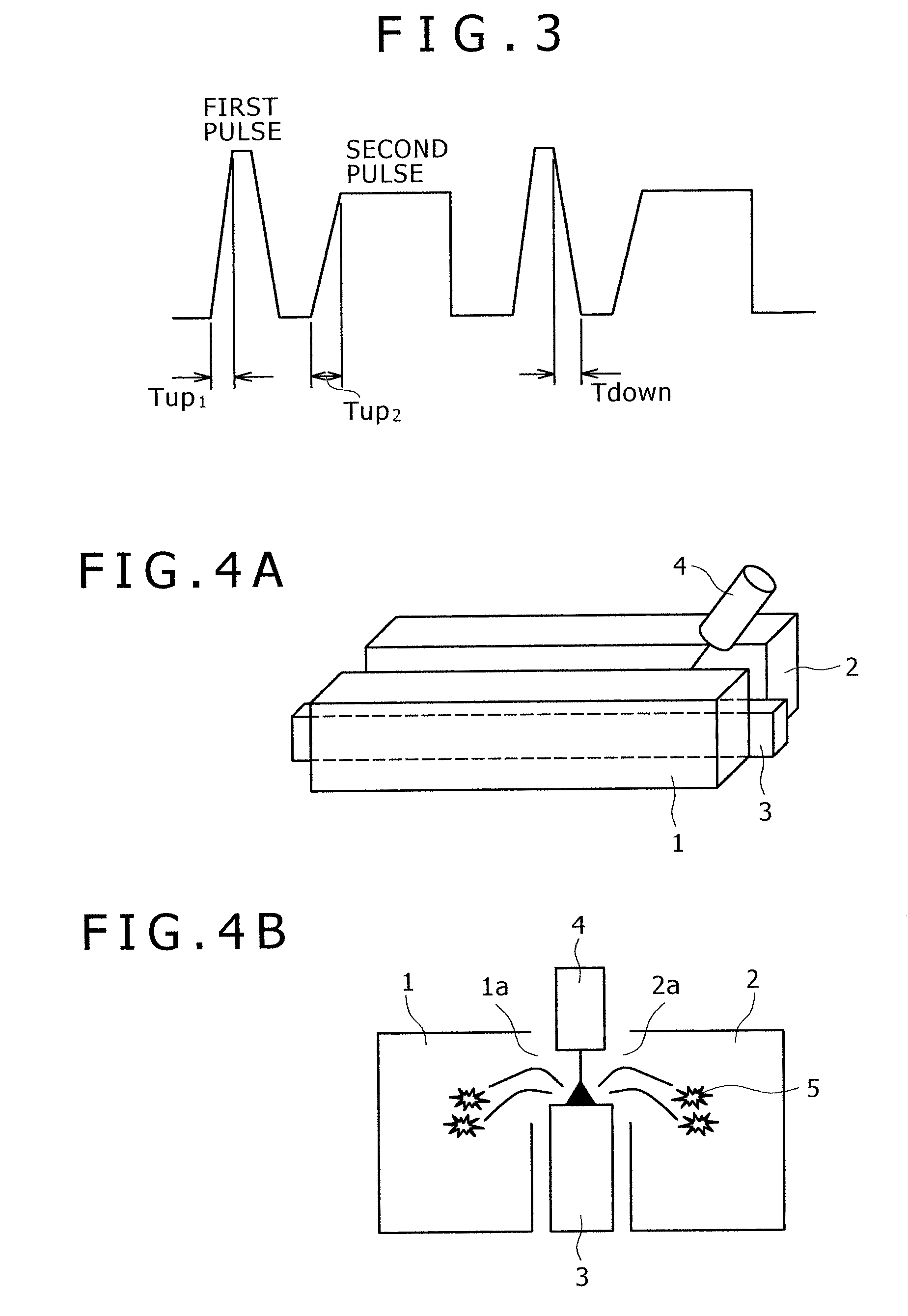
Pulsed arc welding method
ActiveUS20070210048A1Good saveImprove stabilityArc welding apparatusShielding gasCarbon Dioxide / Helium
Disclosed is a pulsed arc welding method using a pulse current of alternately repeating first and second pulses as a weld current, wherein the first pulse and the second pulse have a pulse waveform of a different pulse peak current level and a different pulse width, respectively, and the following conditions are satisfied: peak current of the first pulse (Ip1)=300 to 700A; peak period (Tp1)=0.3 to 5.0 ms; base current Ib1=30 to 200A, base period (Tb1)=0.3 to 10 ms; peak current of the second pulse (Ip2)=200 to 600A; peak period (Tp2)=1.0 to 15 ms; base current (Ib2)=30 to 200A; and base period (Tb2)=3.0 to 20 ms. In this manner, the consumable electrode arc welding using carbon dioxide gas alone or a mixed gas made mainly of carbon dioxide gas as a shield gas can benefit from stabilized welding arc, improved regularity of droplet transfer, and significantly reduced generation rates of spatters and fumes.
Owner:KOBE STEEL LTD
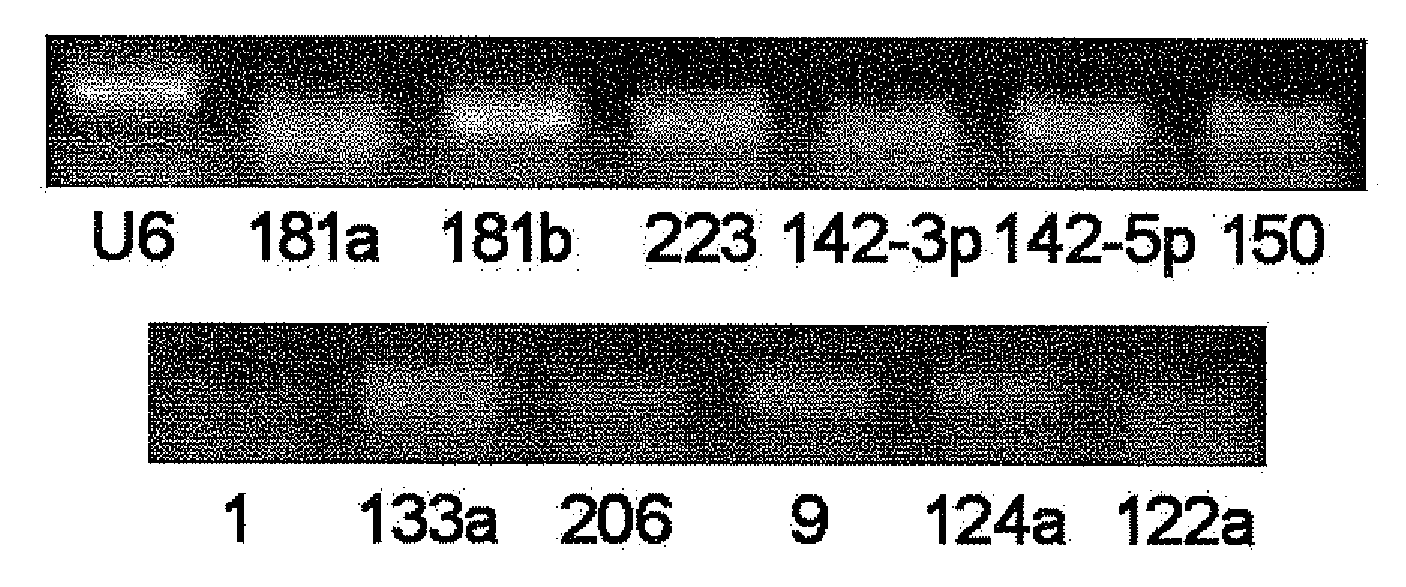
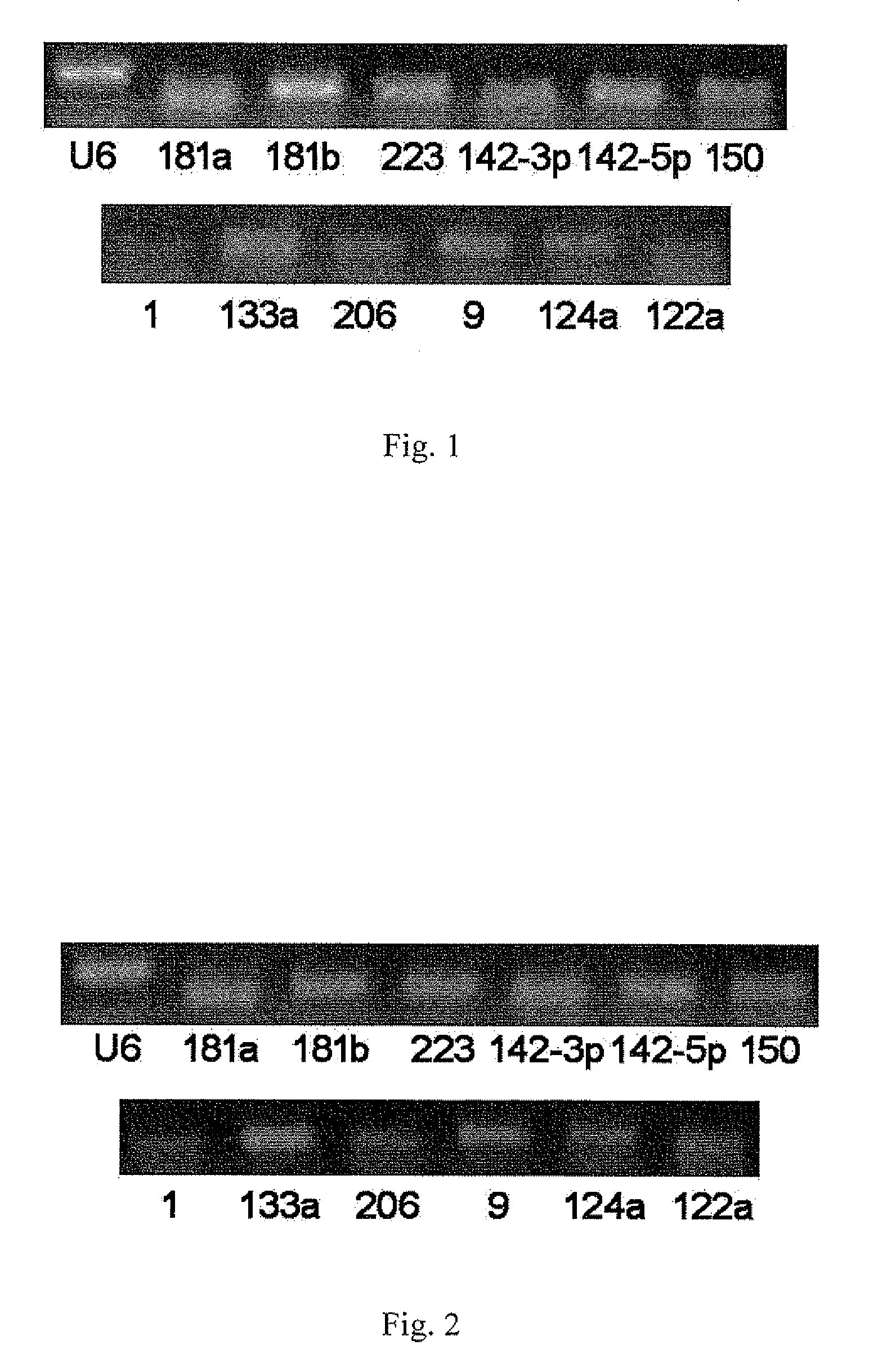
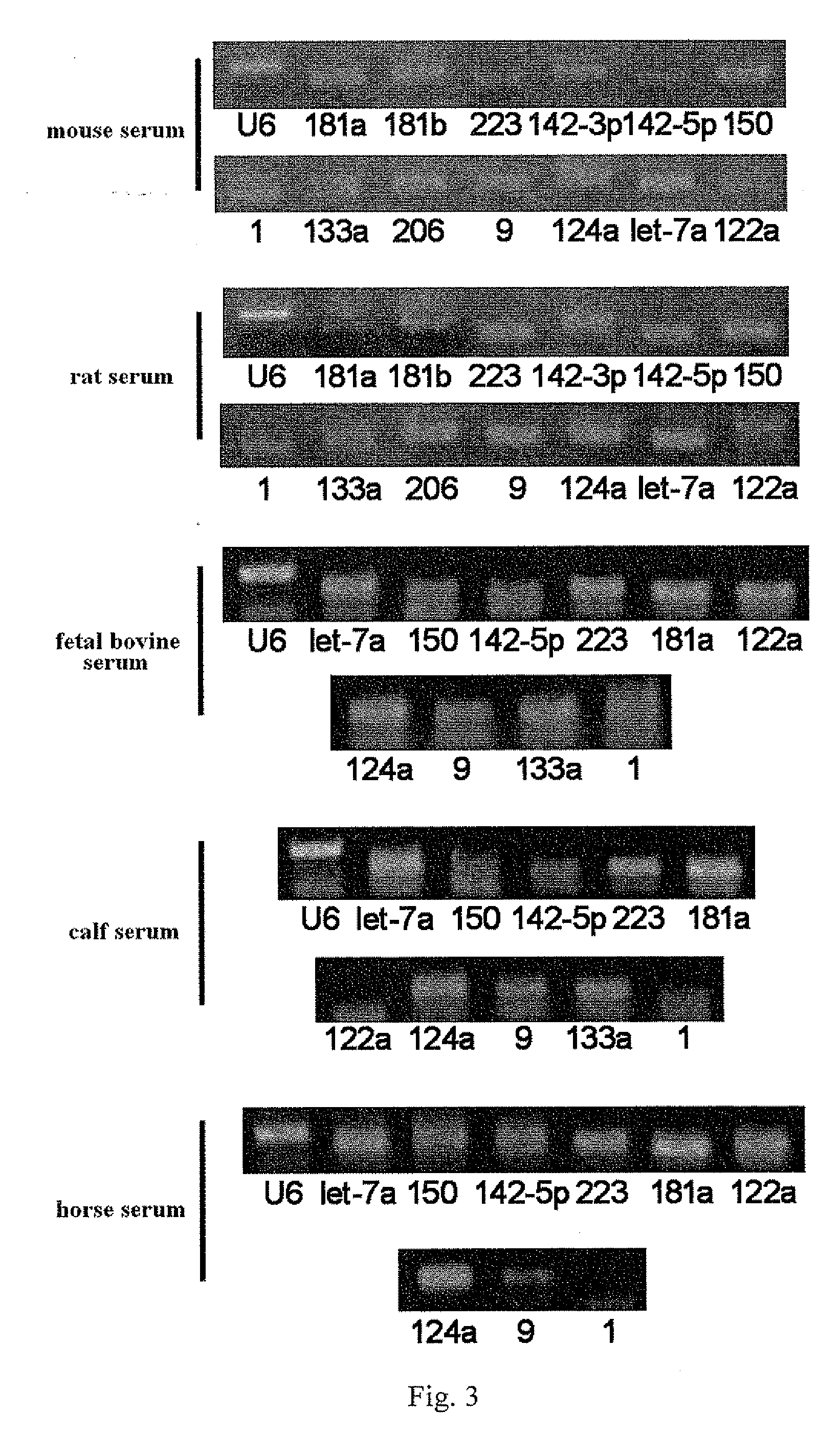
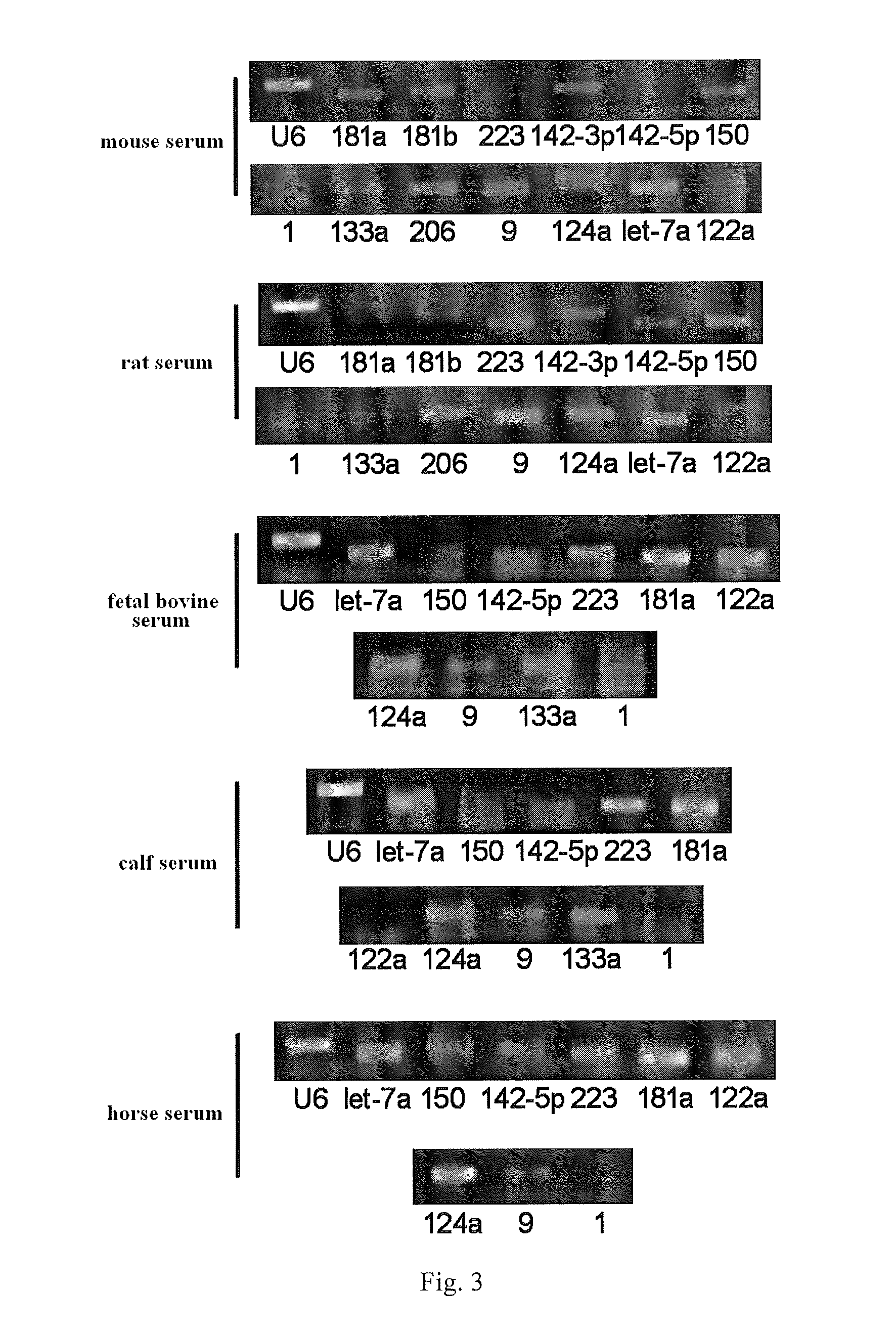
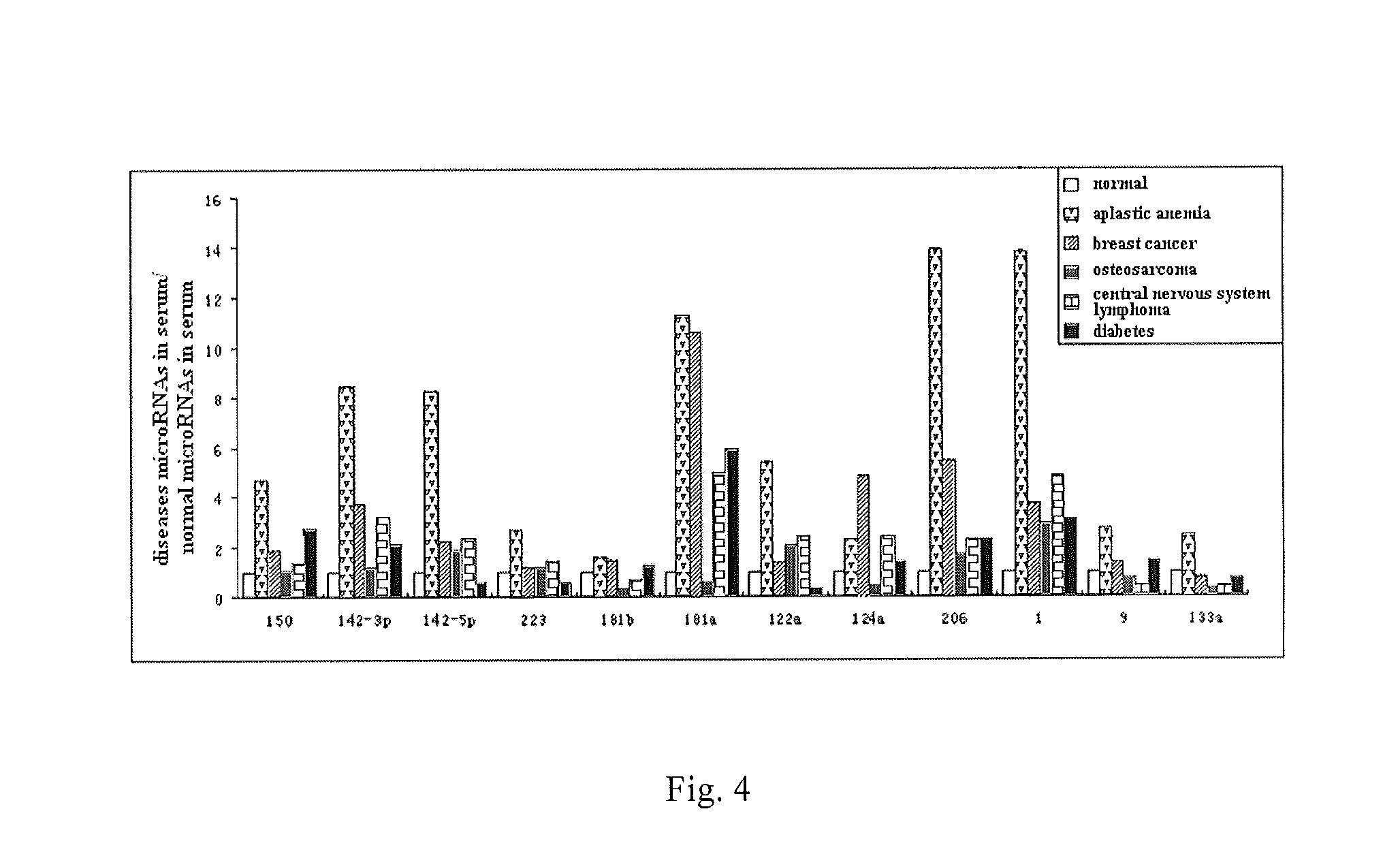
Serum/plasma micronas and uses thereof
InactiveUS20100173288A1Large spectrumHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsUropathyTherapeutic effect
This invention provides a combination of microRNAs for evaluating the physiological and / or pathological condition of a subject, wherein the combination comprises all detectable microRNAs stably existing in the serum / plasma of a subject; and a method for evaluating the physiological and / or pathological condition of a subject, wherein the method includes determining all detectable microRNAs stably existing in the serum / plasma of a subject; and a kit for evaluating the physiological and / or pathological condition of a subject, wherein the kit contains the tools for determining all detectable microRNAs that stably existing in the serum / plasma of a subject; and a biochip for evaluating the physiological and / or pathological condition of a subject, wherein the biochip contains the components for determining all detectable microRNAs stably existing in the serum / plasma of a subject. The aforementioned combination, method, kit and biochip can be used for diagnosis as well as differentially diagnosis of diseases including various tumors; various acute / chronic infectious diseases, e.g. viral diseases such as viral influenza, viral hepatitis, AIDS, SARS, bacterial diseases such as tuberculosis, bacterial pneumonia, and other acute / chronic infectious diseases caused by various pathogenic microorganisms; other acute / chronic diseases such as diseases of respiratory system, diseases of immune system, diseases of blood and hematopoietic system, diseases of circulatory system such as cardio-cerebrovascular diseases, metabolic diseases of endocrine system, diseases of digestive system, diseases of nervous system, diseases of urinary system, diseases of reproductive system and diseases of locomotor system, prediction of complications occurrence and malignant diseases relapse, evaluation of therapeutic effects, screening of pharmaceutical active ingredients, assessment of drug efficacy as well as forensic authentication and prohibited drug inspection and the like, possessing a number of advantages such as extensive detection spectrum, high sensitivity, low cost, convenience for sampling, ease for sample preservation, etc. The said method can be widely used in work related to general survey of diseases and so on, improve the low-specificity and low-sensitivity caused by individual differences which single markers are difficult to overcome, significantly increasing the clinical detection rate of diseases, all of which make it become an effective means for diagnosing diseases in an early phase.
Owner:JIANGSU MINGMA BIOTECH
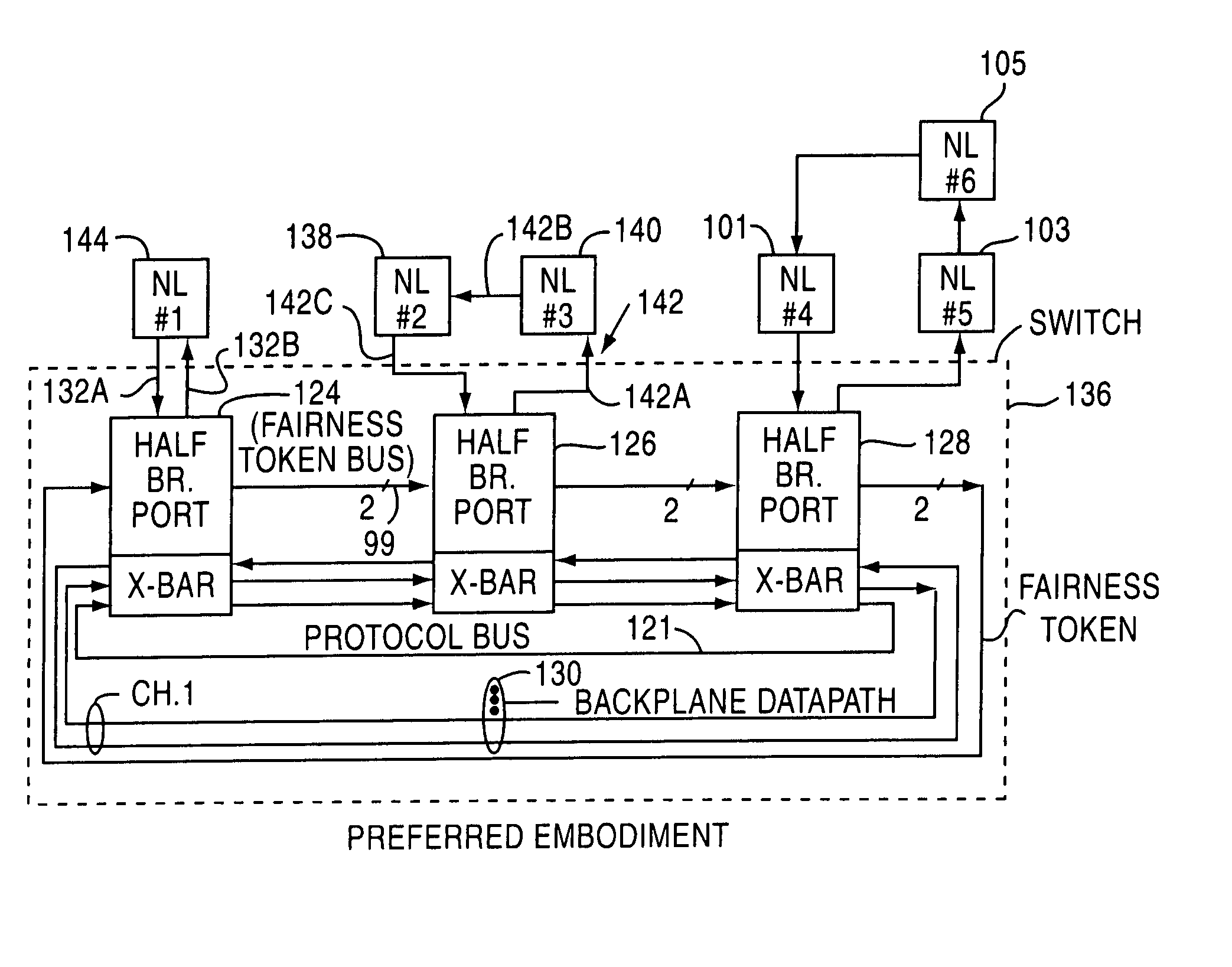
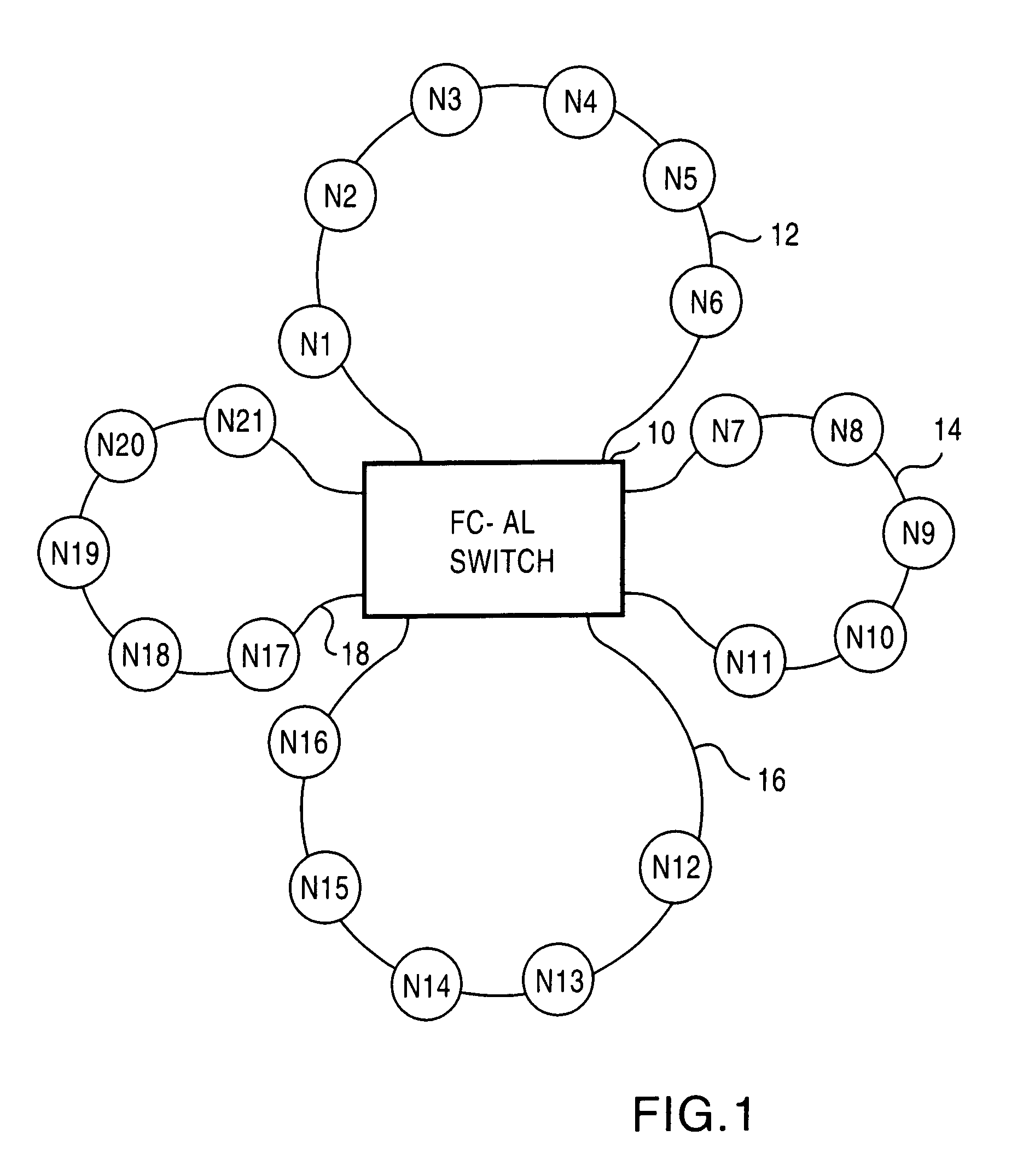
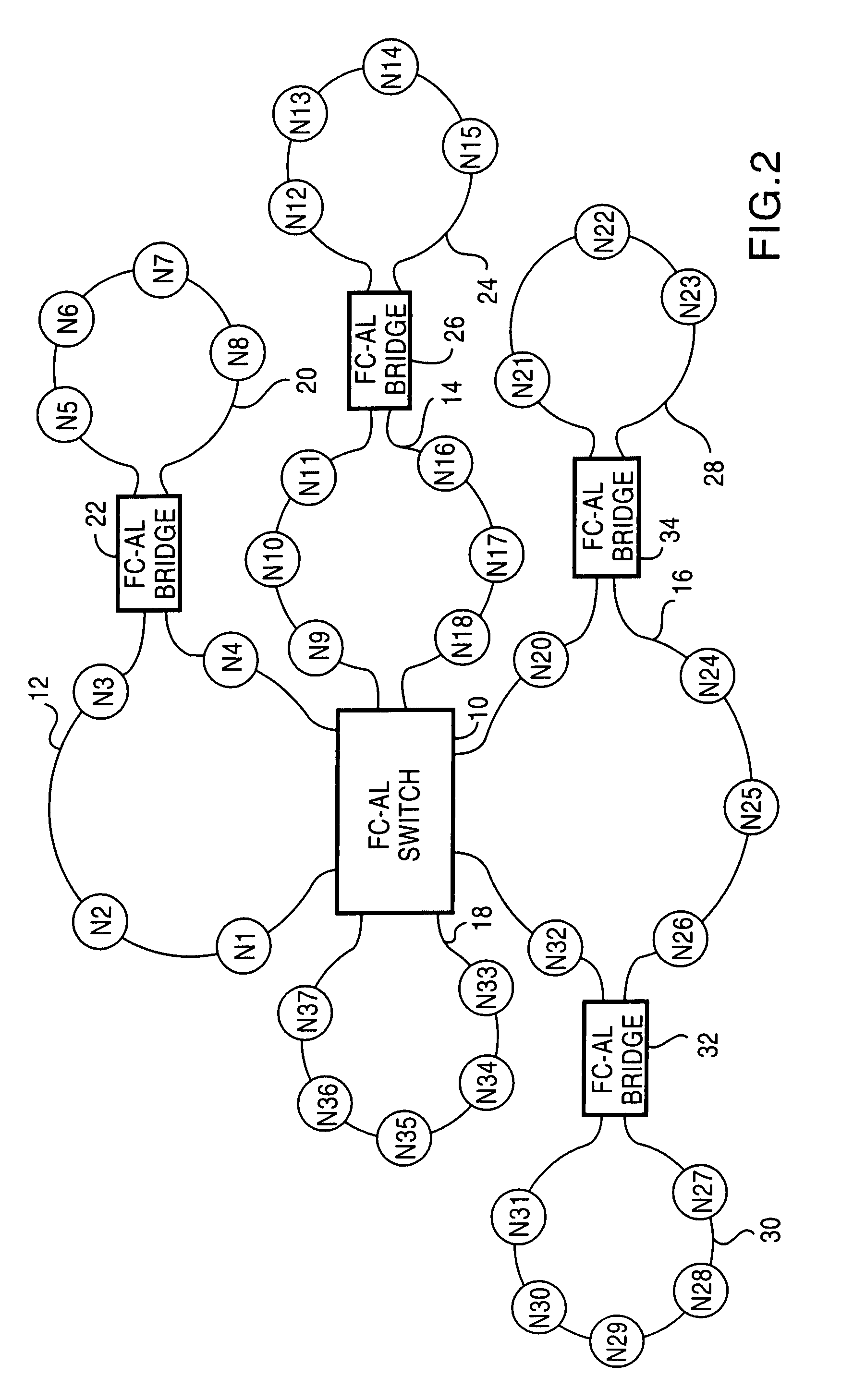
Fibre channel arbitrated loop bufferless switch circuitry to increase bandwidth without significant increase in cost
InactiveUS7362769B2Good saveImprove network throughputTime-division multiplexLoop networksBackplaneCrossbar switch
Owner:AVAGO TECH INT SALES PTE LTD
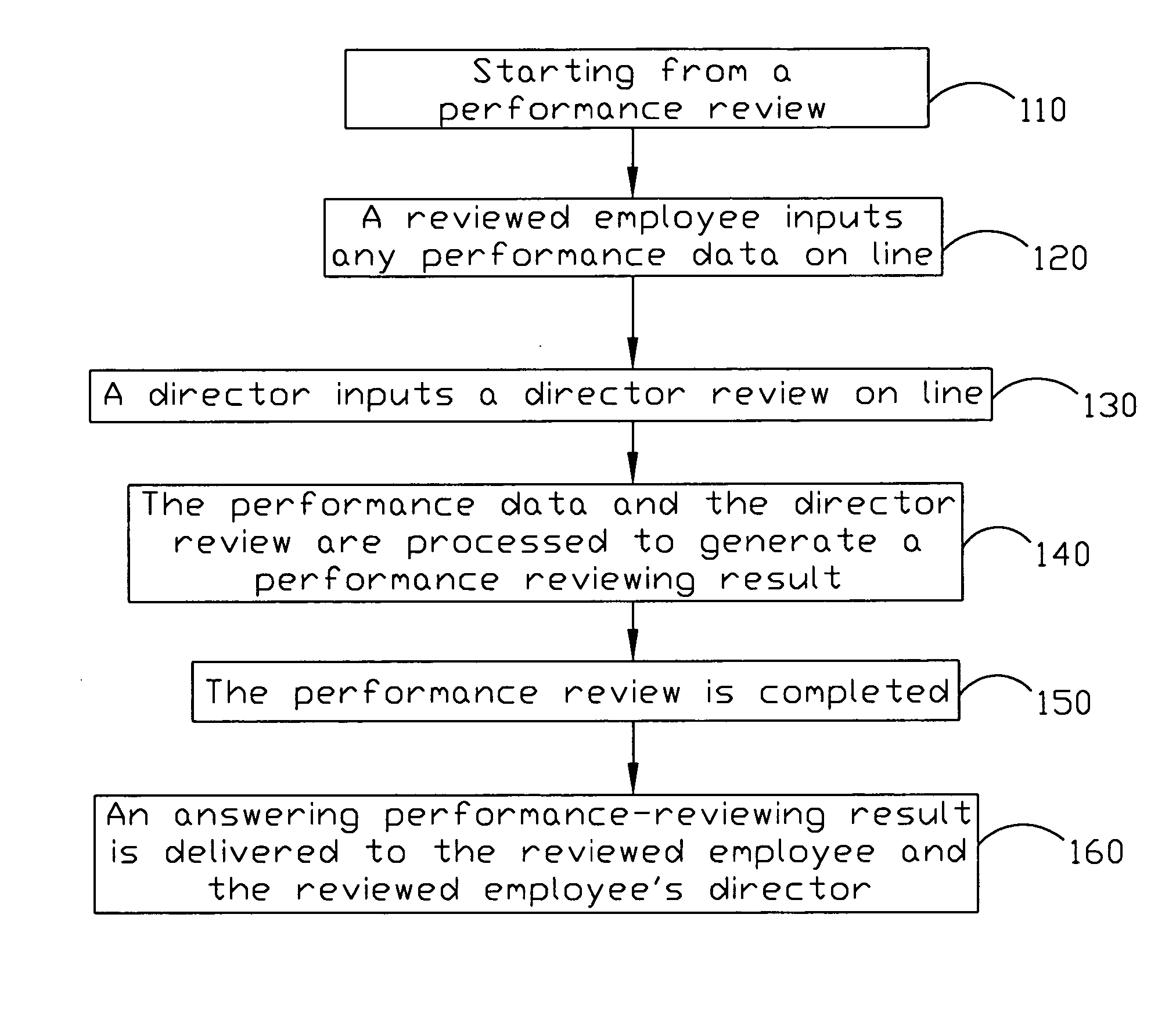
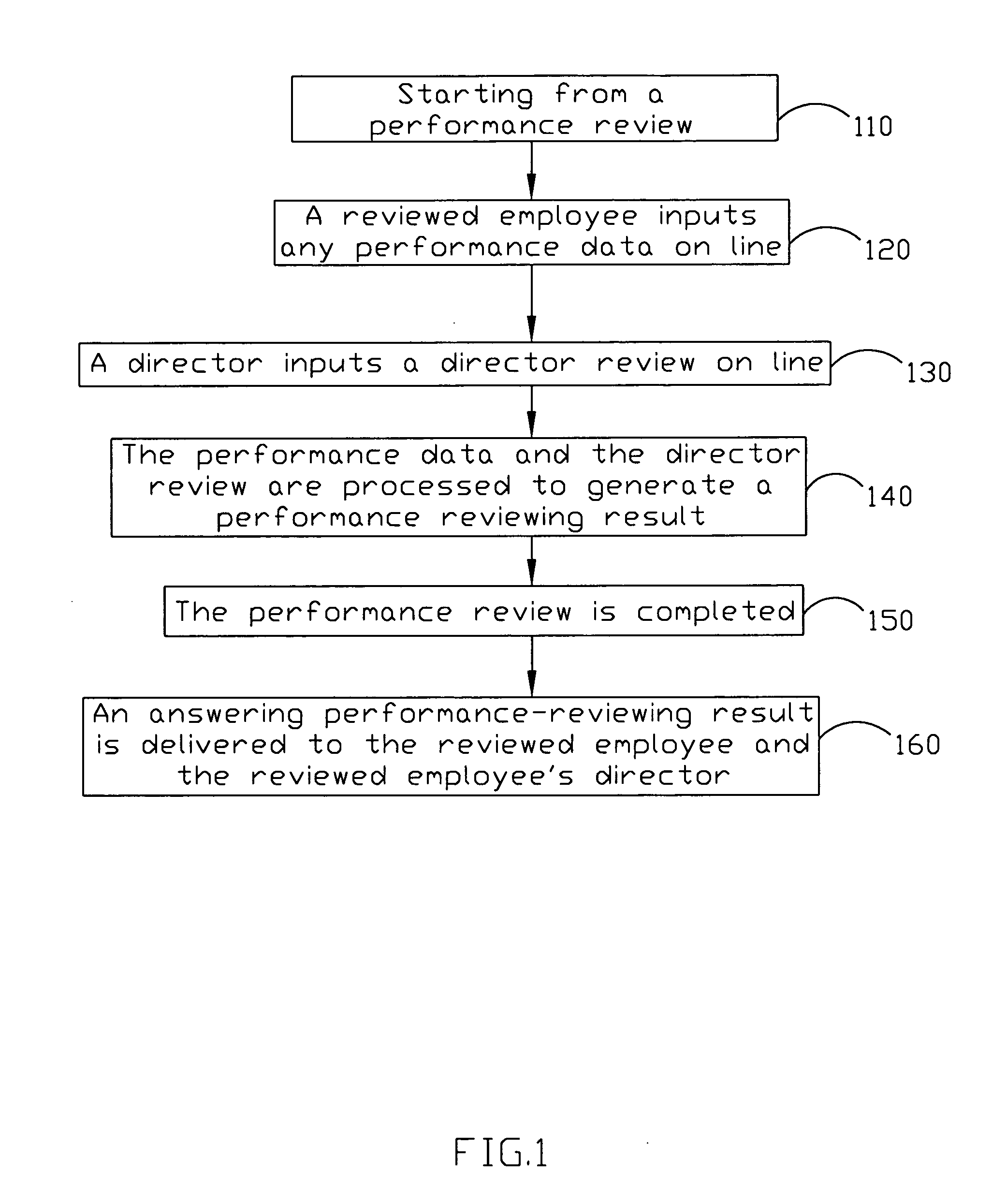
Employee performance reviewing method and system
InactiveUS20050021391A1Good saveNo paper usedOffice automationSpecial data processing applicationsConfidentialityEmployee Performance Appraisal
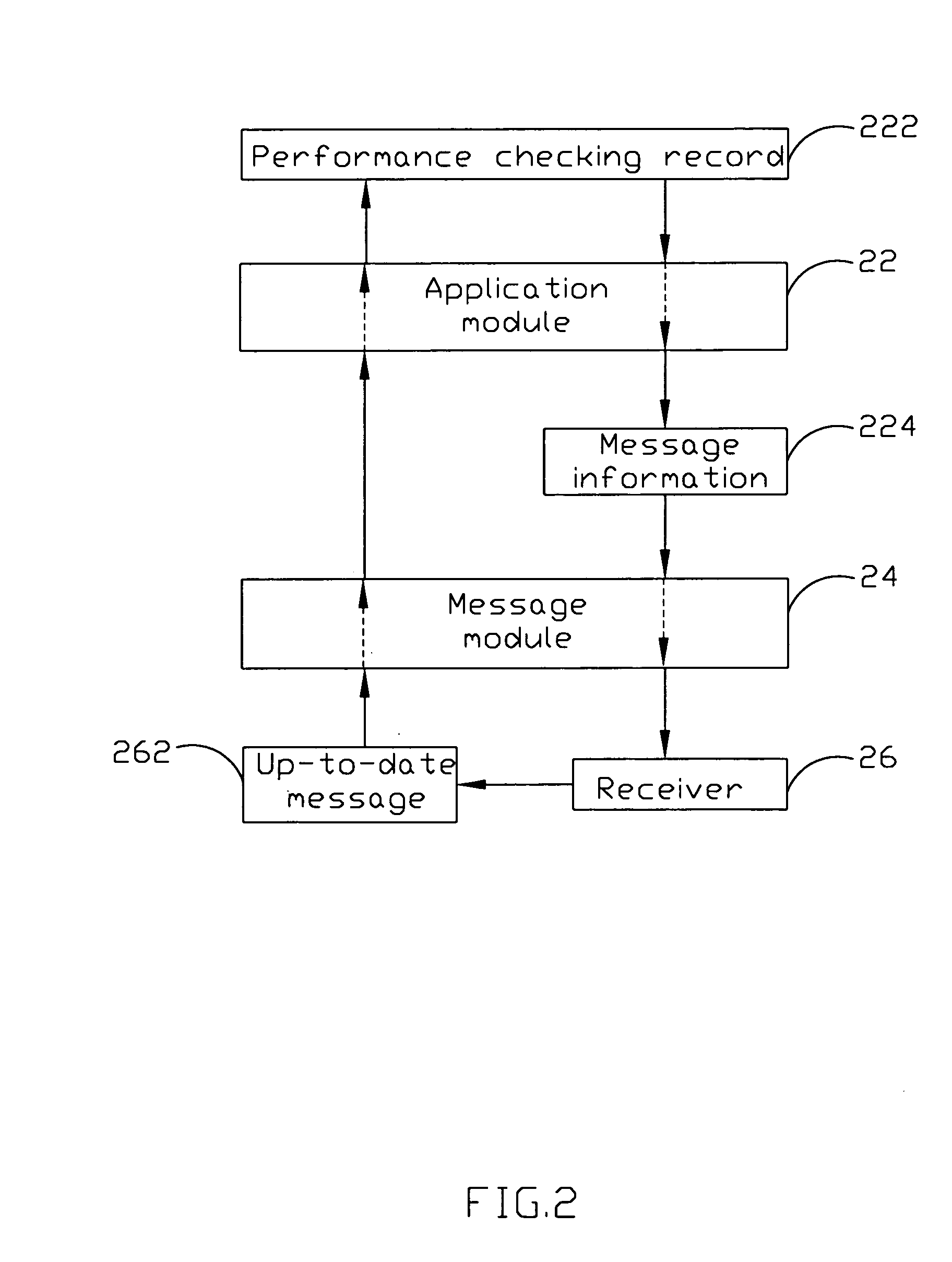
The present invention discloses an employee performance reviewing system, comprising a performance checking record, an application module for generating a message information and assigning a dispatching time depending on the performance checking record, and a message module for sending the message information to at least a receiver and receiving up-to-date messages from the receivers. The up-to-date message is generated according the message information and added into the performance checking record. By using of requesting the following-up checkers assessing the performances, the objective of procedure control can be reviewed. The present invention can further comprise an interface module and an encryption-decryption module to achieve the objective of the confidentiality and security of the data with cooperating a security mechanism.
Owner:VIA TECH INC
Game system that uses collection card, game machine, and storage medium that stores game program
InactiveUS20050009610A1Easy to copyData augmentationVideo gamesSpecial data processing applicationsCard readerHuman–computer interaction
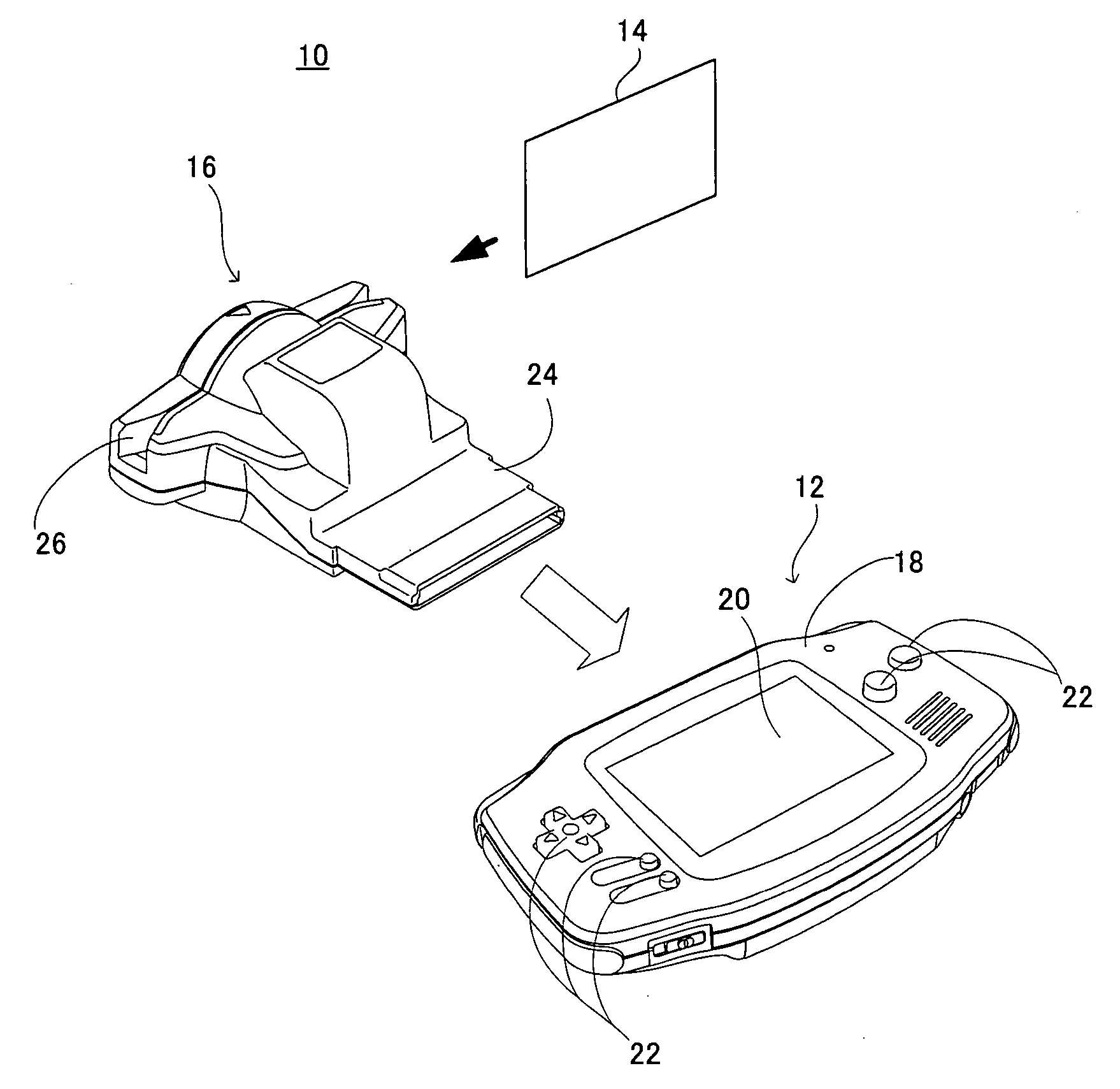
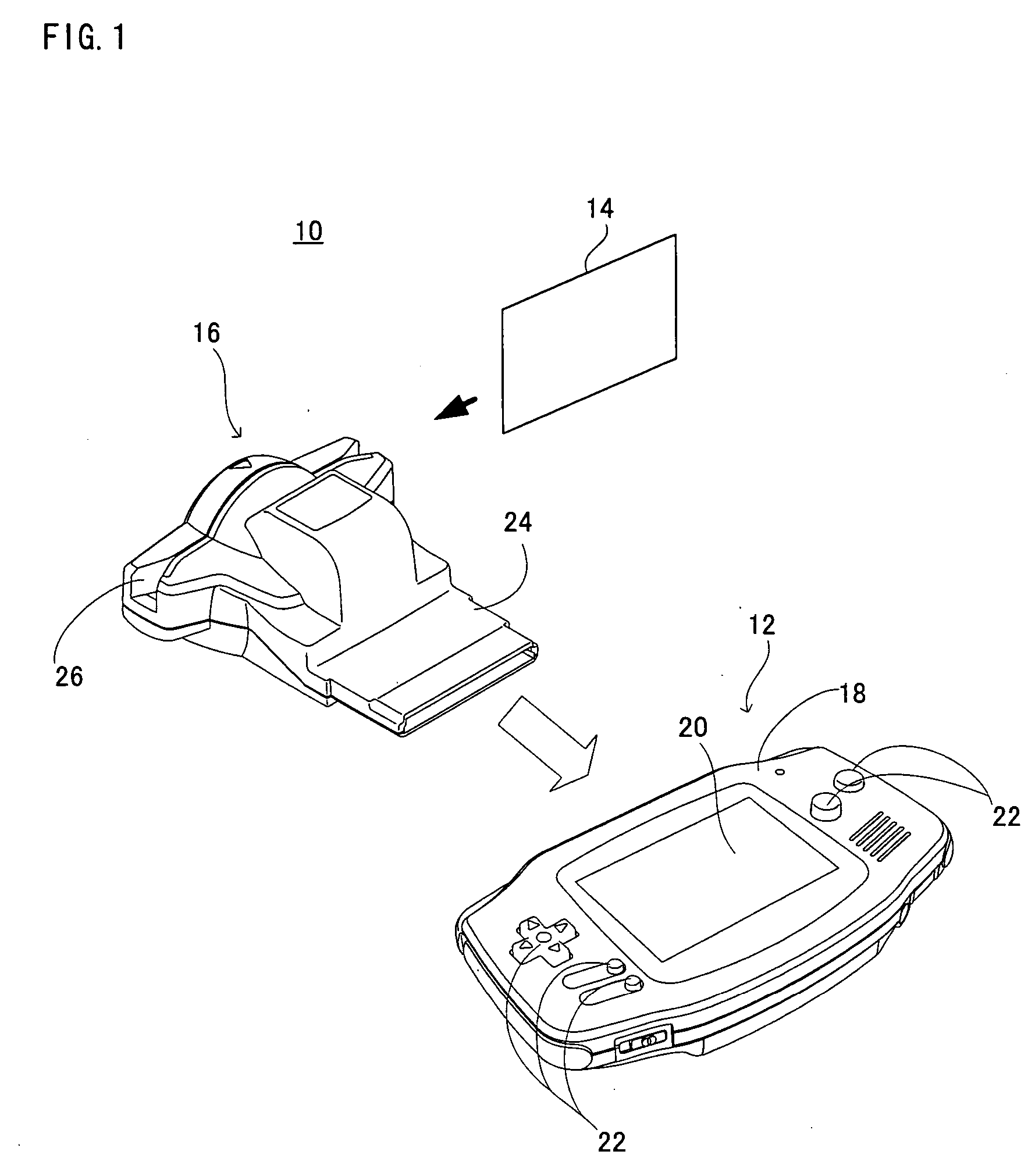
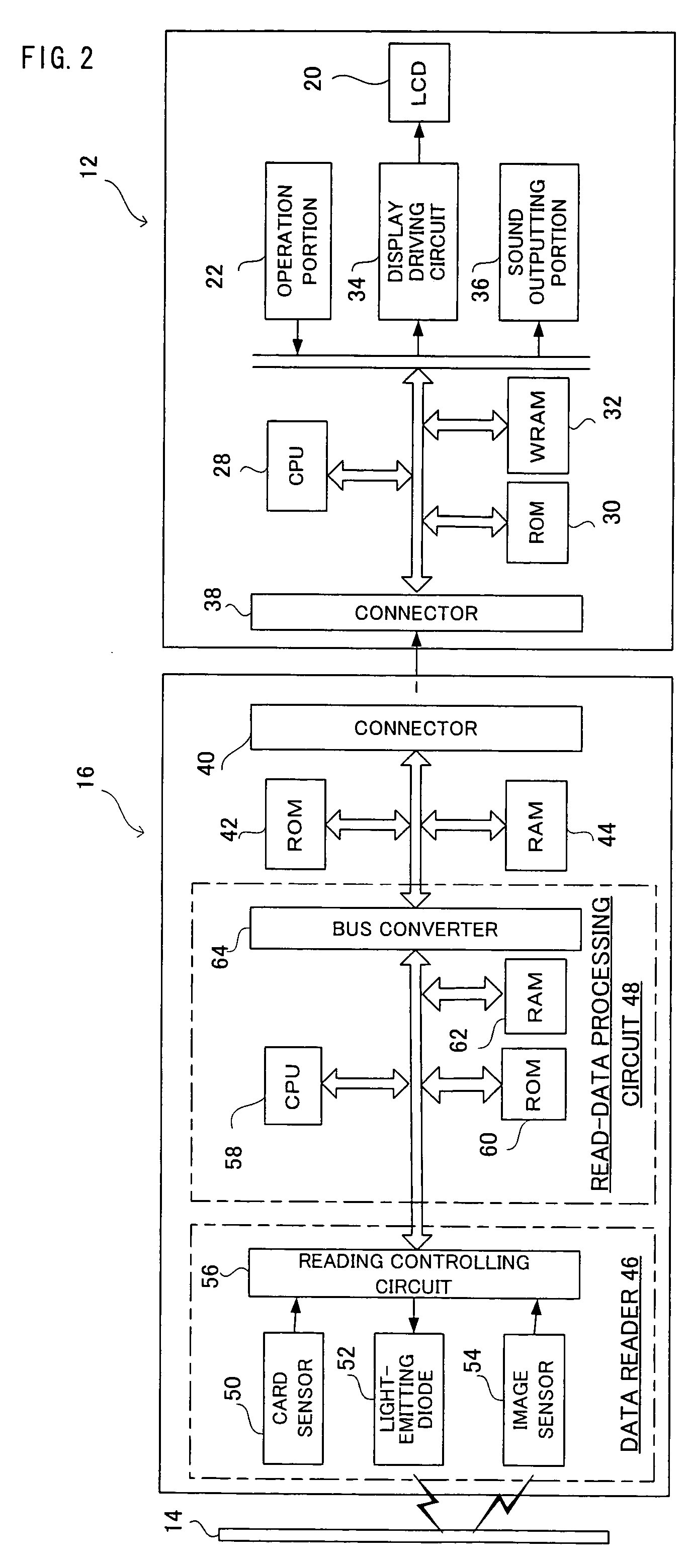
A game system includes a game machine, and is a system for playing an interlocking game between a collection card and the game machine. In the collection card, game data for the interlocking game is recorded in a mechanically readable manner, and this game data is read out by a card reader, which is temporarily stored in a WRAM of the game machine. A game process is applied to the game data in accordance with a game program and an operation input by a player. Furthermore, it is determined whether or not this game achieves a predetermined condition, that is, it is determined whether or not the game is cleared, for example, and as long as the predetermined condition is achieved, the game data and clear information of the game are saved in a backup RAM 44.
Owner:NINTENDO CO LTD
Method of improved controlling of an ink jet printer, and ink jet printer
InactiveUS20070024650A1Easy to optimizeEasy to controlOther printing apparatusComputer printingInk printer
A method for improved controlling of an ink jet printer includes measuring the amount of ink dosed to at least one printhead of the ink jet printer, measuring the amount of ink droplets released by at least one nozzle of the printhead, and determining the amount of a ink contained by the printhead by using the amounts measured. A computer program and a computer running the computer program are adapted to carry out the method. Moreover, an ink jet printer is adapted to perform the method.
Owner:OCE TECH
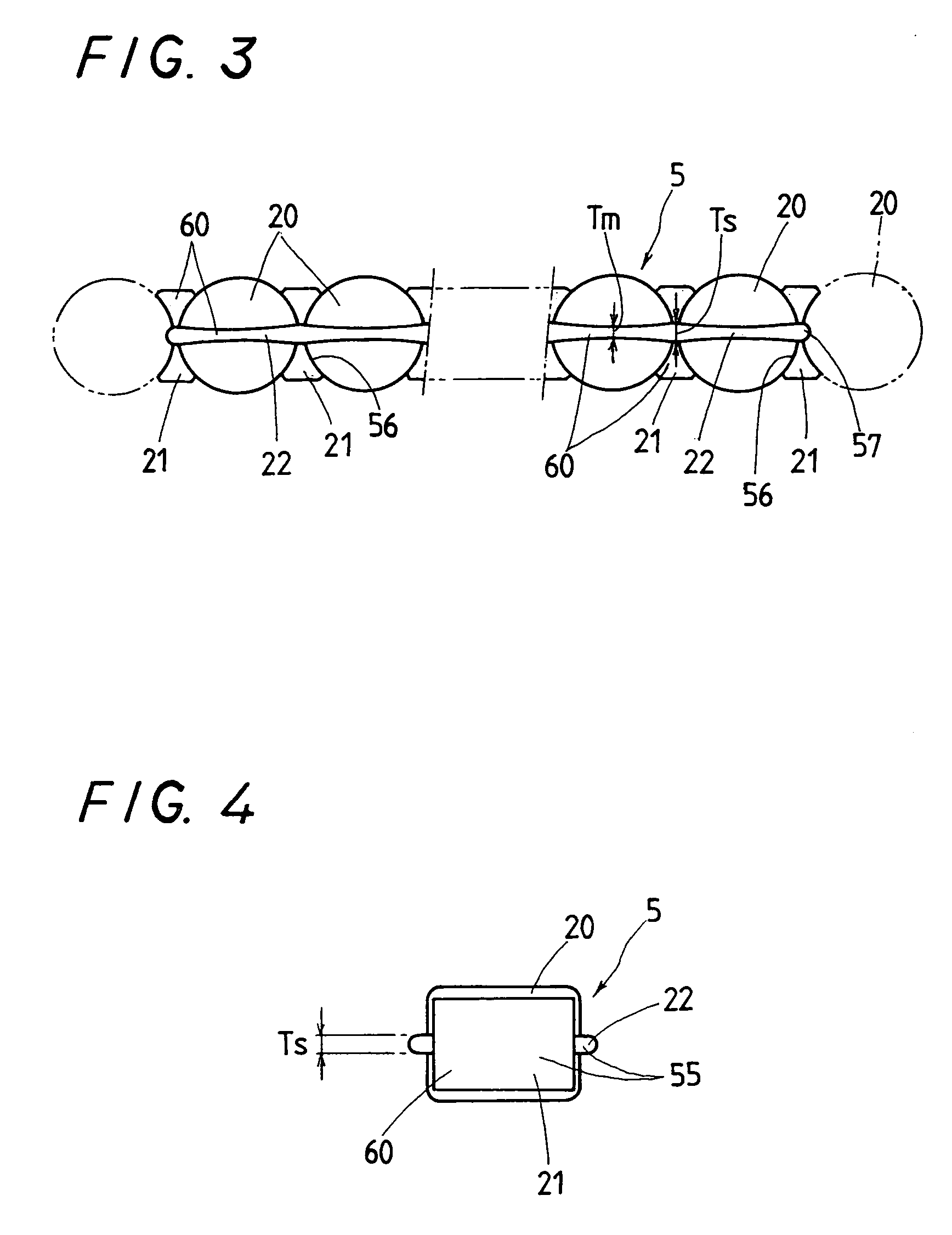
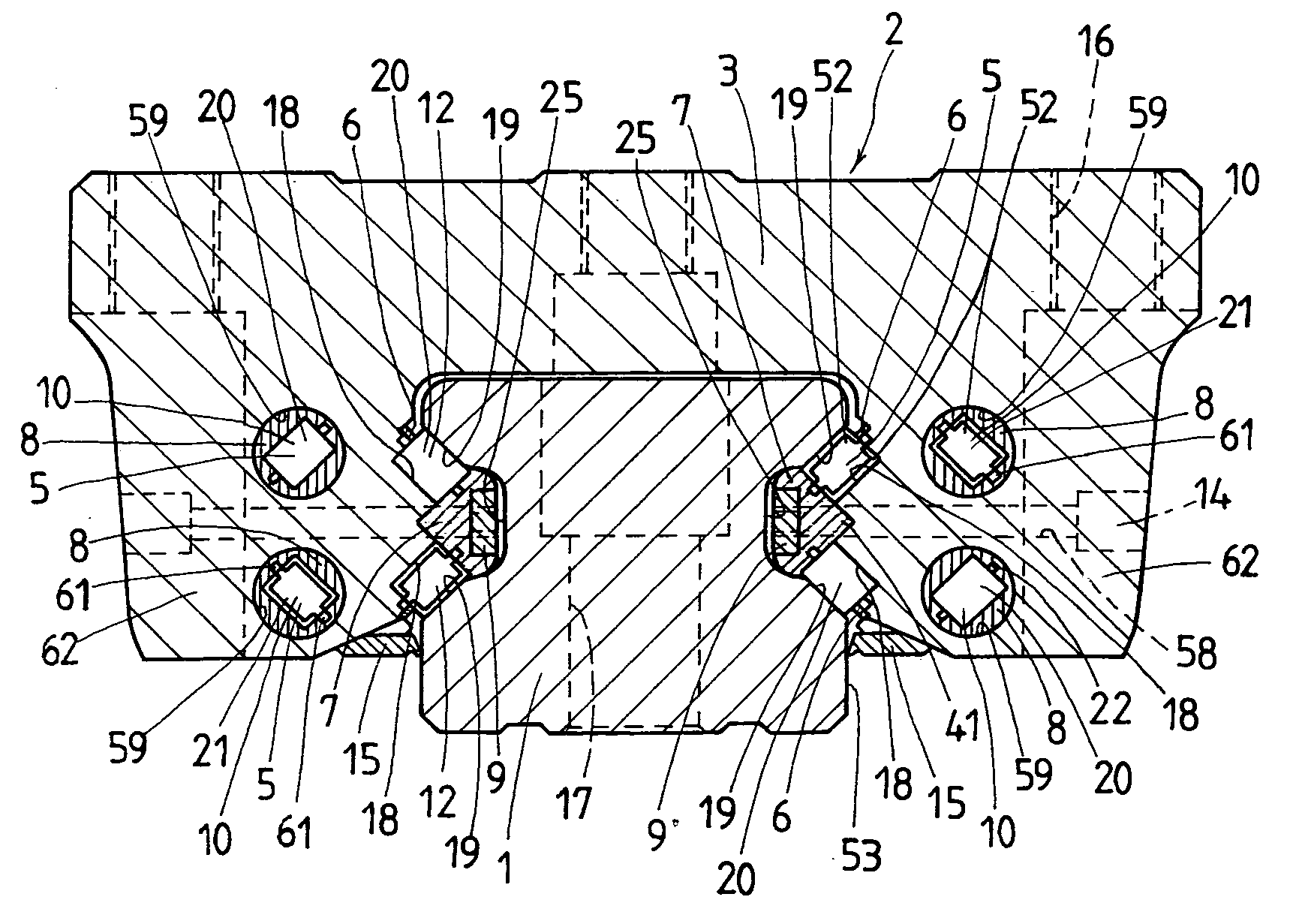
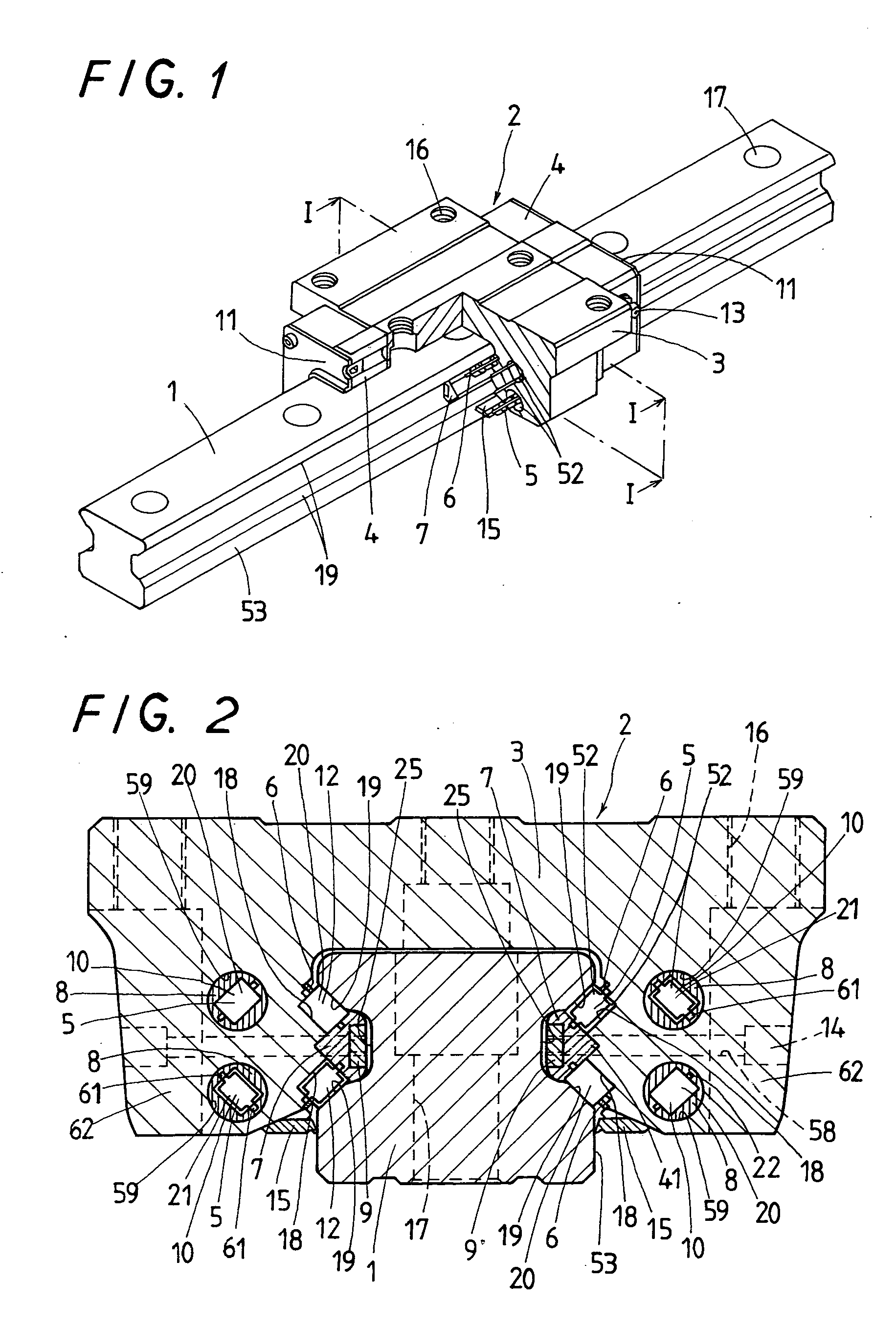
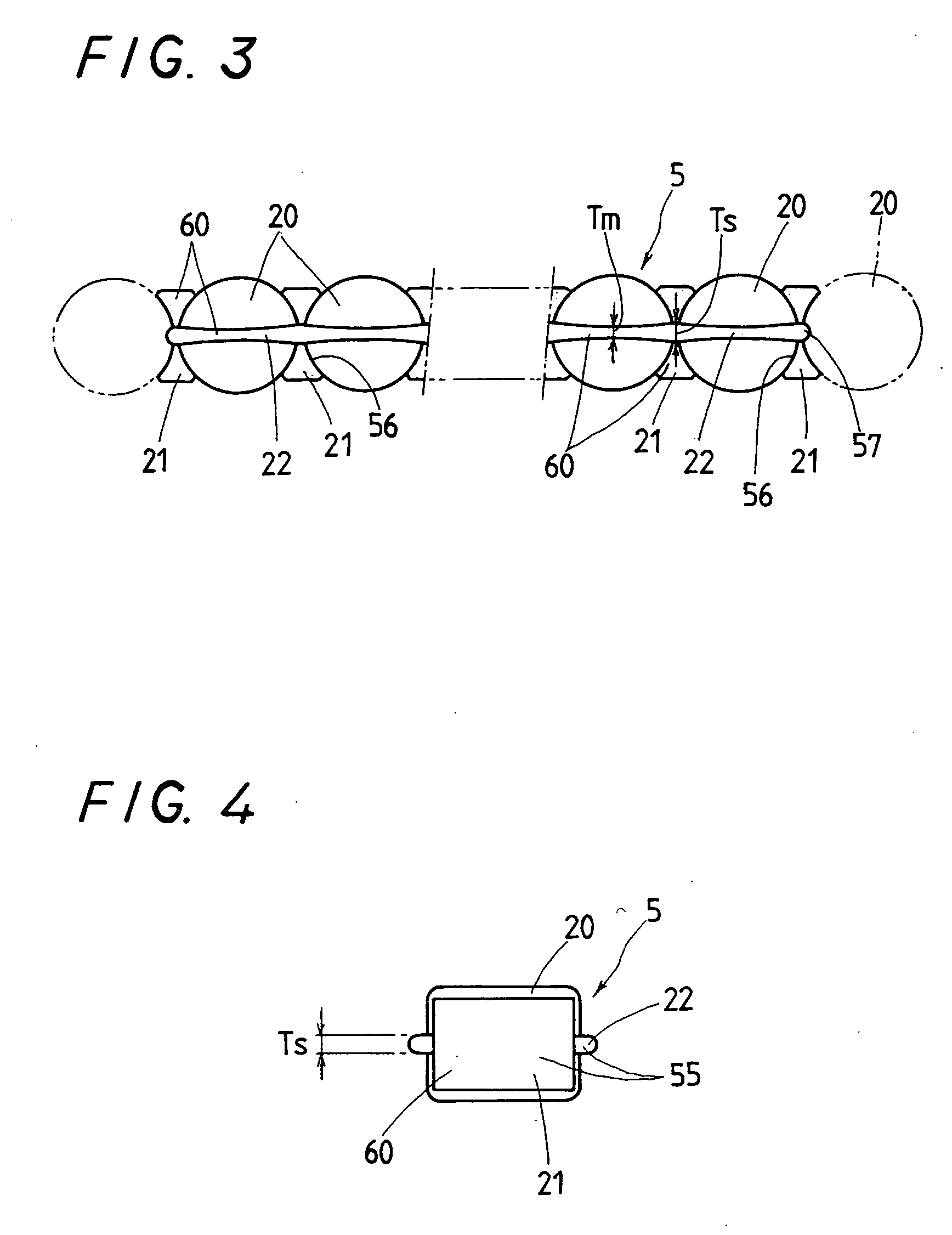
Linear motion guide unit
A linear motion guide unit is disclosed in which there is provided a retainer band making it possible to easily fit a roller chain with rollers traveling through a recirculation circuit into a load way of the recirculation circuit, thereby making sure of smooth recirculation of the roller chain. Cooperation of the retainer band with a retainer plate helps guide links of the roller chain in a load way of the recirculation circuit, keeping the roller chain against running out of the load way thereby ensuring smooth movement of the roller chain throughout the recirculation circuit.
Owner:NIPPON THOMPSON
Sample plate for MALDI mass spectrometry and process for manufacture of the same
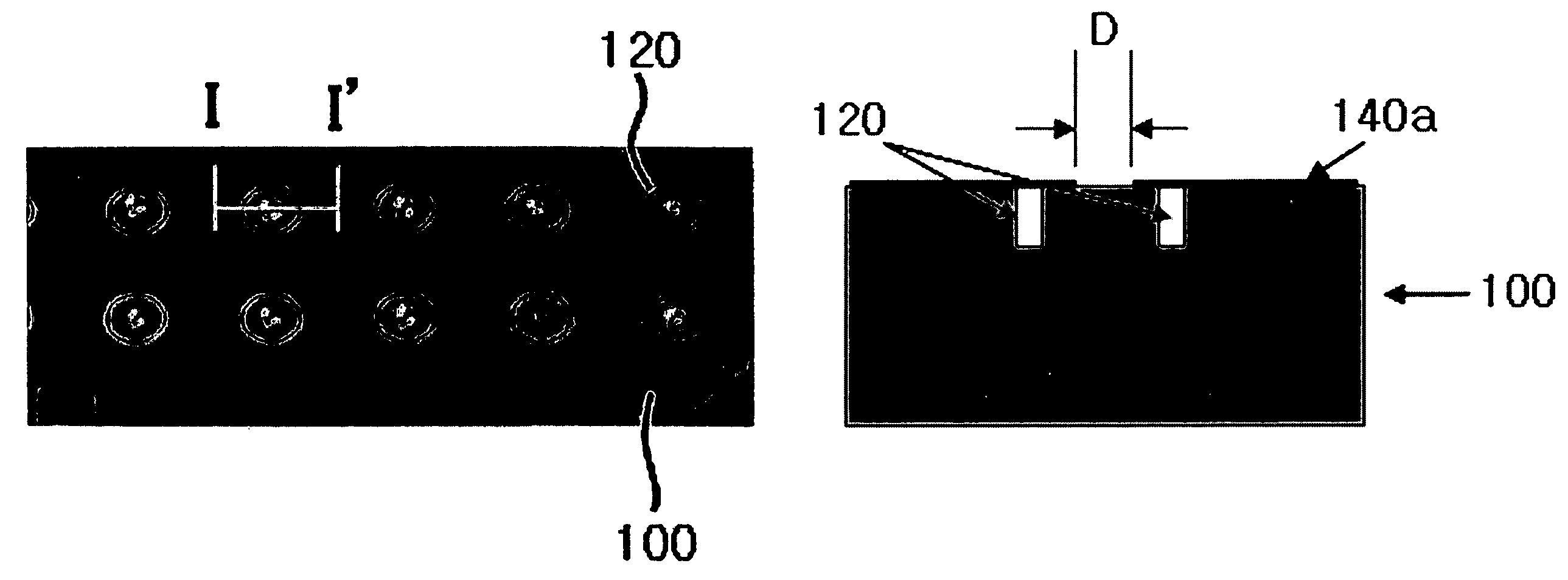
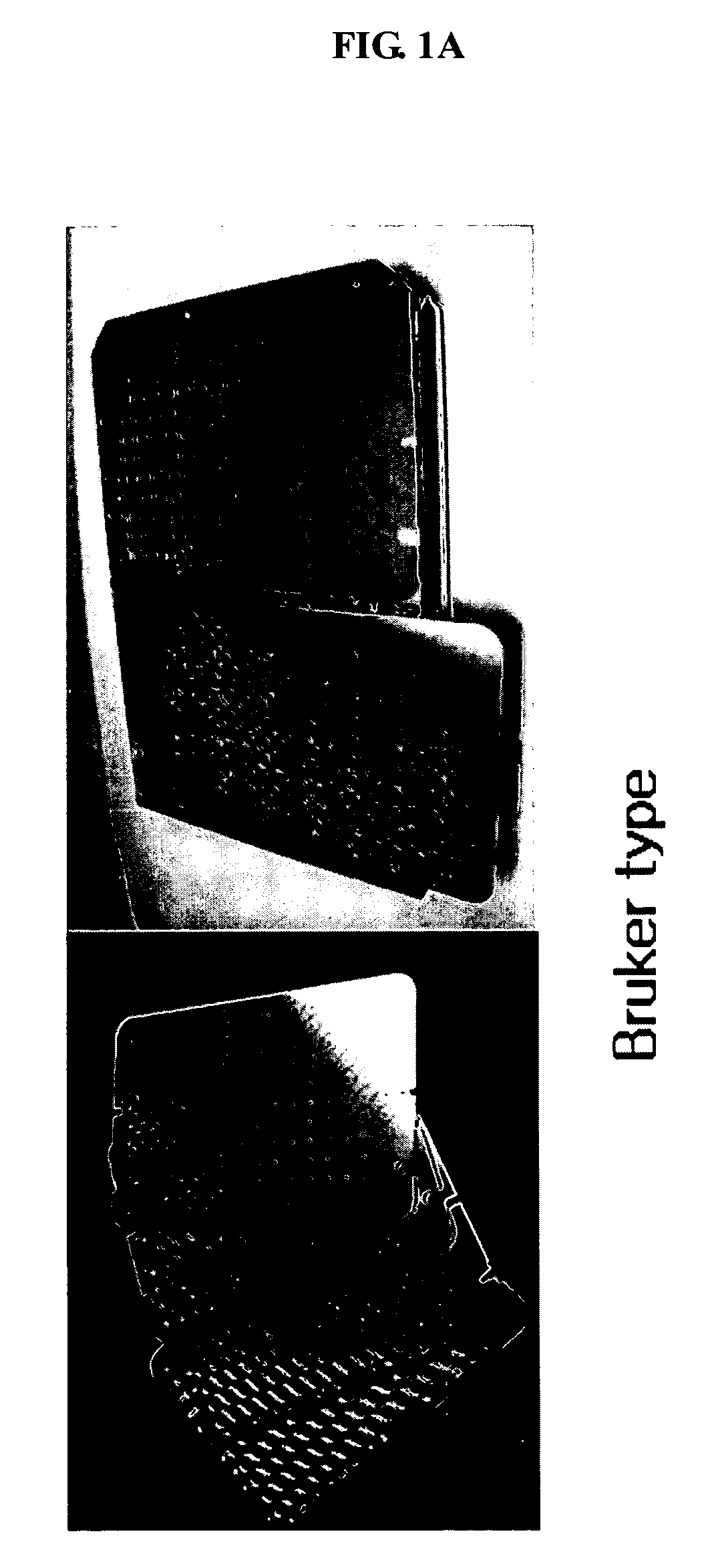
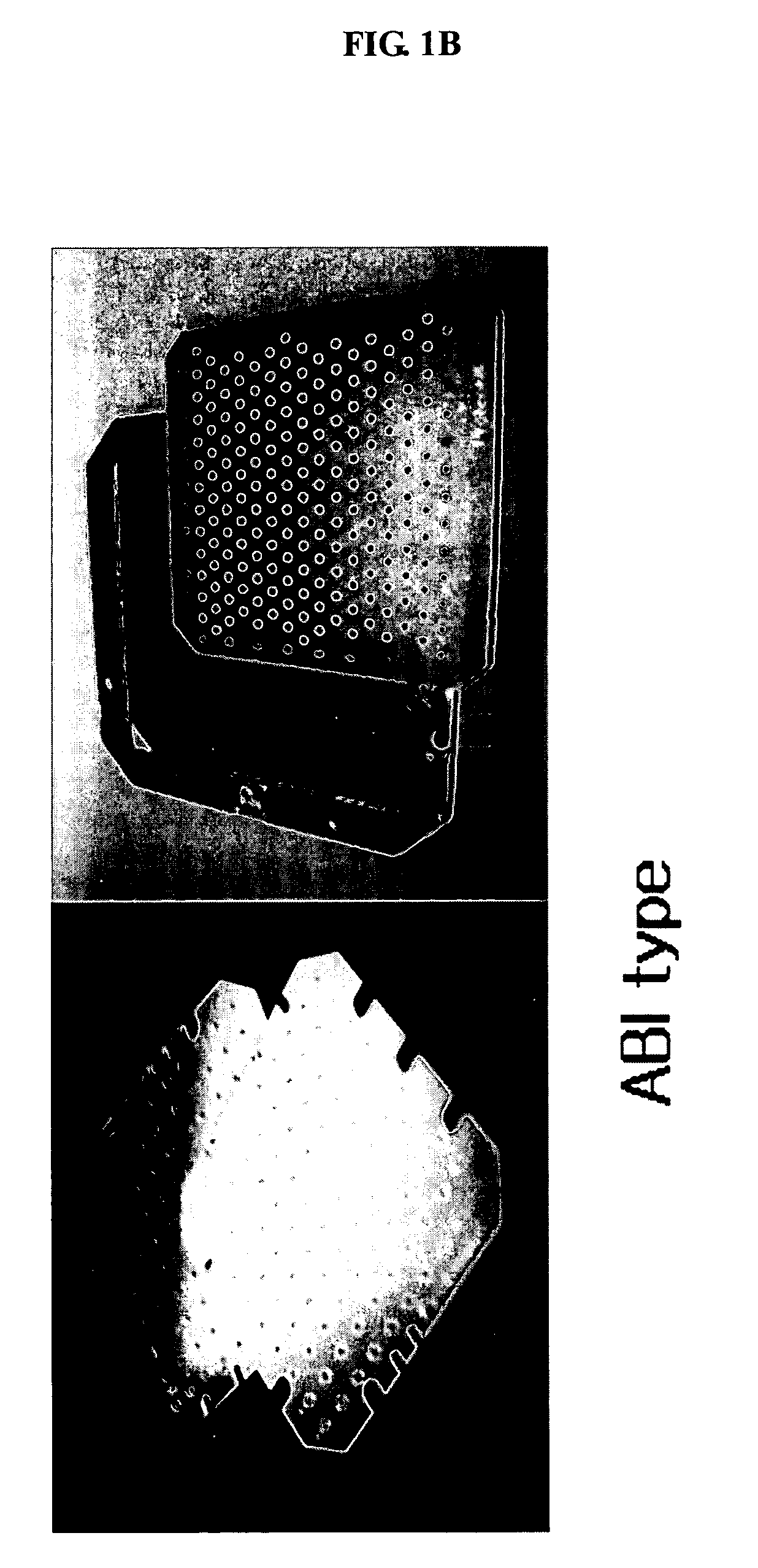
ActiveUS20070075241A1Good adhesionEasy detachmentParticle separator tubesSamplingCost effectivenessMass Spectrometry-Mass Spectrometry
The present invention relates to a sample microfocusing plate useful in MALDI mass spectrometry having a patterned hydrophobic organosilane coating layer and at least a central portion formed on the surface and a process for manufacturing and using the sample microfocusing plate. The sample microfocusing plate can rapidly dry the solvent contained in samples leading to efficient sample analysis, and can be prepared by cost effectiveness.
Owner:ASTA
Vacuum sealed container and method for using thereof
ActiveUS20190009967A1Easily substancePreservative and examinable environmentDomestic cooling apparatusLighting and heating apparatusEngineeringMechanical engineering
The present application discloses a vacuum sealed container, including a container body, a container lid, a vacuum generator, at least one magnifier and an indicator. The container body includes a first wall and a bottom surface, wherein the first wall and the bottom surface define an accommodation space. The container lid is coupleable to the container body. The vacuum generator is coupled to the container lid to evacuate fluid from the accommodation space. The at least one magnifier is coupled to the container body. The indicator is coupled to the container. A method for using the aforementioned vacuum sealed container is also disclosed.
Owner:LUNG ALEX
Method for cell preservation by use of biocompatible particles
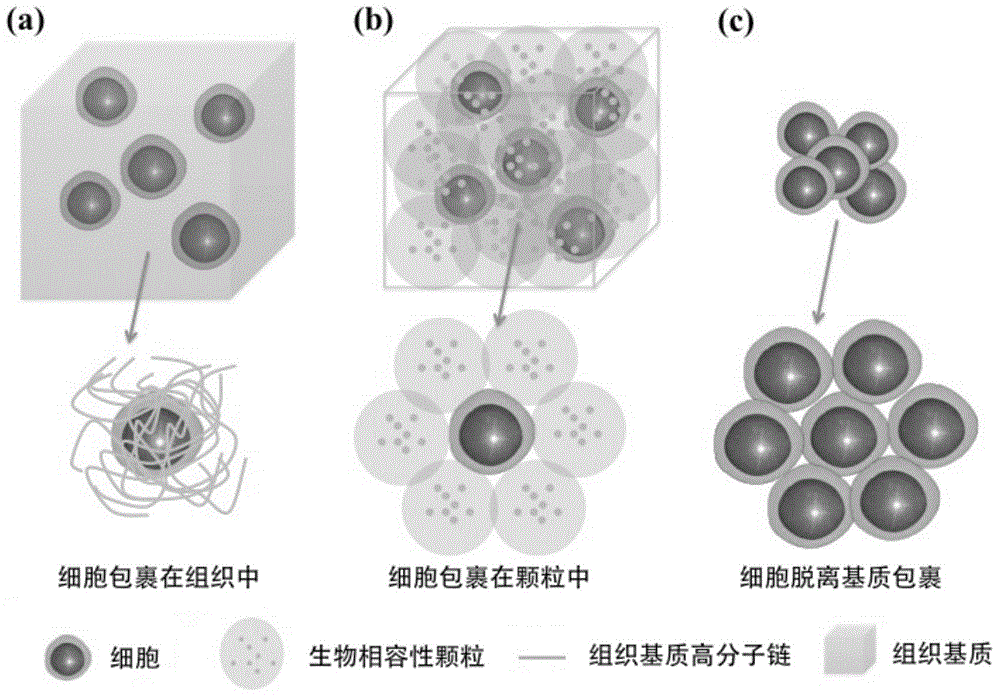
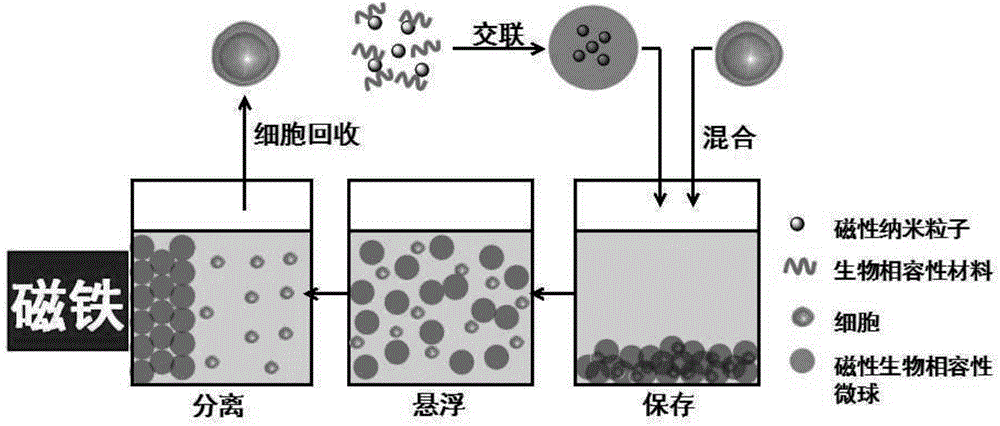
ActiveCN104920339AEfficient transportImprove storage efficiencyDead animal preservationOn/in organic carrierMagnetic separationChemistry
The invention relates to a method for cell preservation by use of biocompatible particles. The method is characterized in that physical mixing and centrifugal enrichment are conducted on hydrogel particles of a pure biocompatible material and cells, and the hydrogel particles are stacked to wrap the cells. The method is also characterized in that hydrogel particles, added with magnetic nano-particles, of a pure biocompatible material and cells are subjected to physical mixing and then centrifugal enrichment, the magnetic hydrogel particles are stacked to wrap the cells, and when separation is required, the magnetic biocompatible particles are separated through magnetic separation to obtain the needed cells. The biocompatible material particles are 10 nm-1000 [mu]m, the compatible particles and the cells are uniformly mixed according to any appropriate proportion and subjected to centrifugal enrichment at 500-2000 rpm for storage. The pure physical method for particle-stacking cell preservation of the biocompatible material in the noncryogenic environment is adopted for the first time, so that a novel, simple, convenient and effective method is provided for cell preservation and transport at different temperatures and different bad degrees.
Owner:TIANJIN UNIV
Linear motion guide unit
ActiveUS20050018933A1Good saveEasy to assembleRoller bearingsLinear bearingsLinear motionEngineering
A linear motion guide unit is disclosed in which there is provided a retainer band making it possible to easily fit a roller chain with rollers traveling through a recirculation circuit into a load way of the recirculation circuit, thereby making sure of smooth recirculation of the roller chain. Cooperation of the retainer band with a retainer plate helps guide links of the roller chain in a load way of the recirculation circuit, keeping the roller chain against running out of the load way thereby ensuring smooth movement of the roller chain throughout the recirculation circuit.
Owner:NIPPON THOMPSON
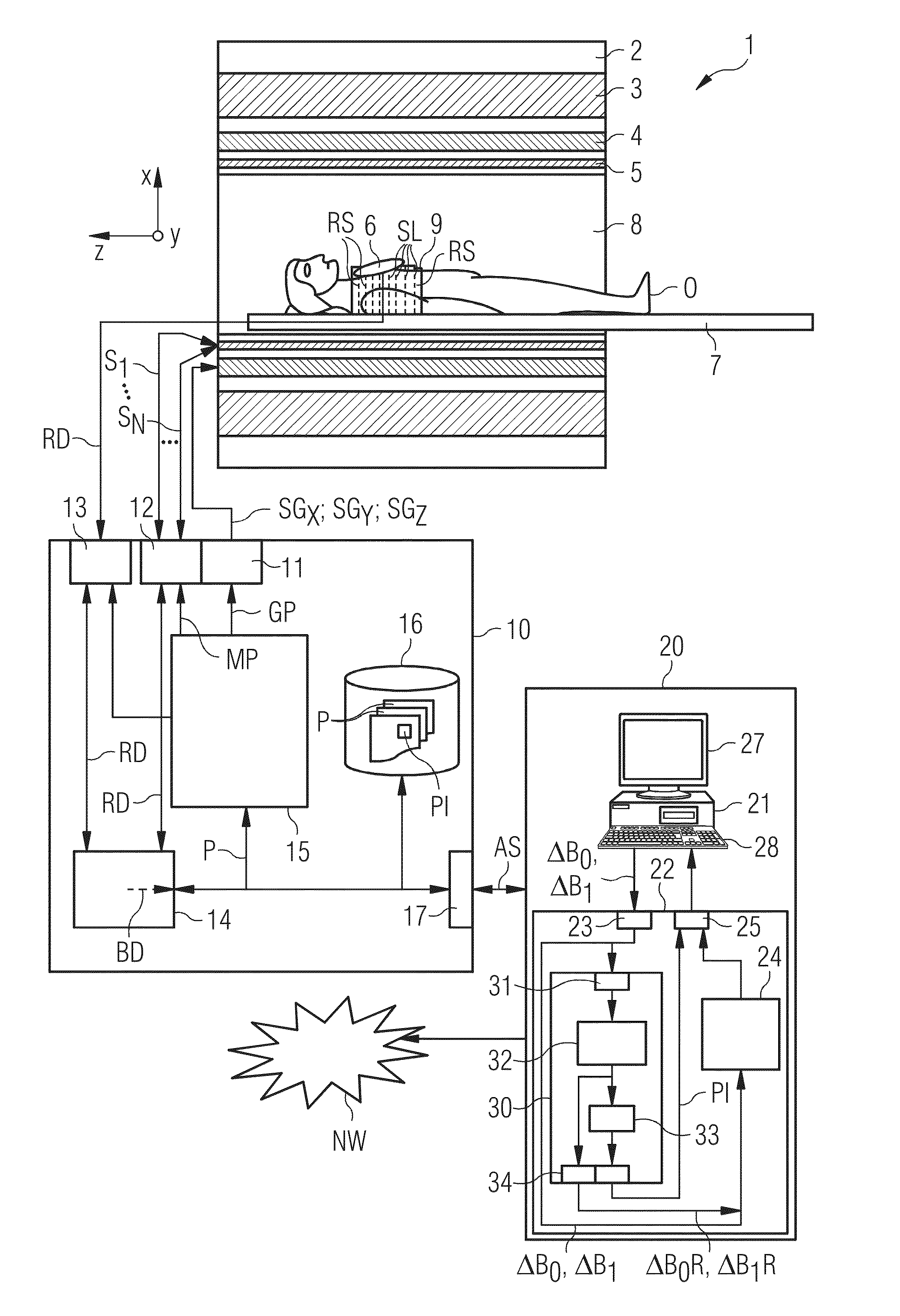
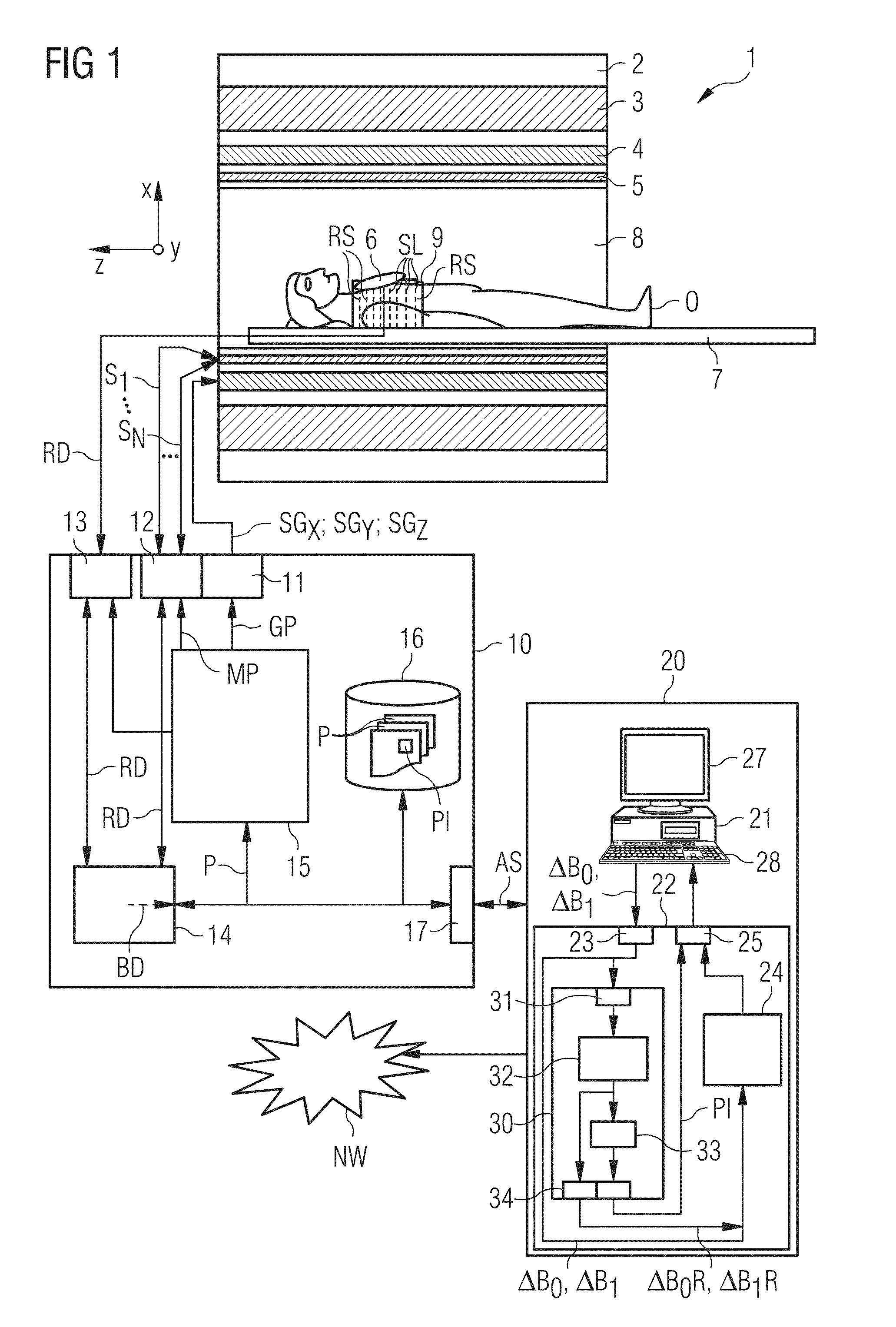
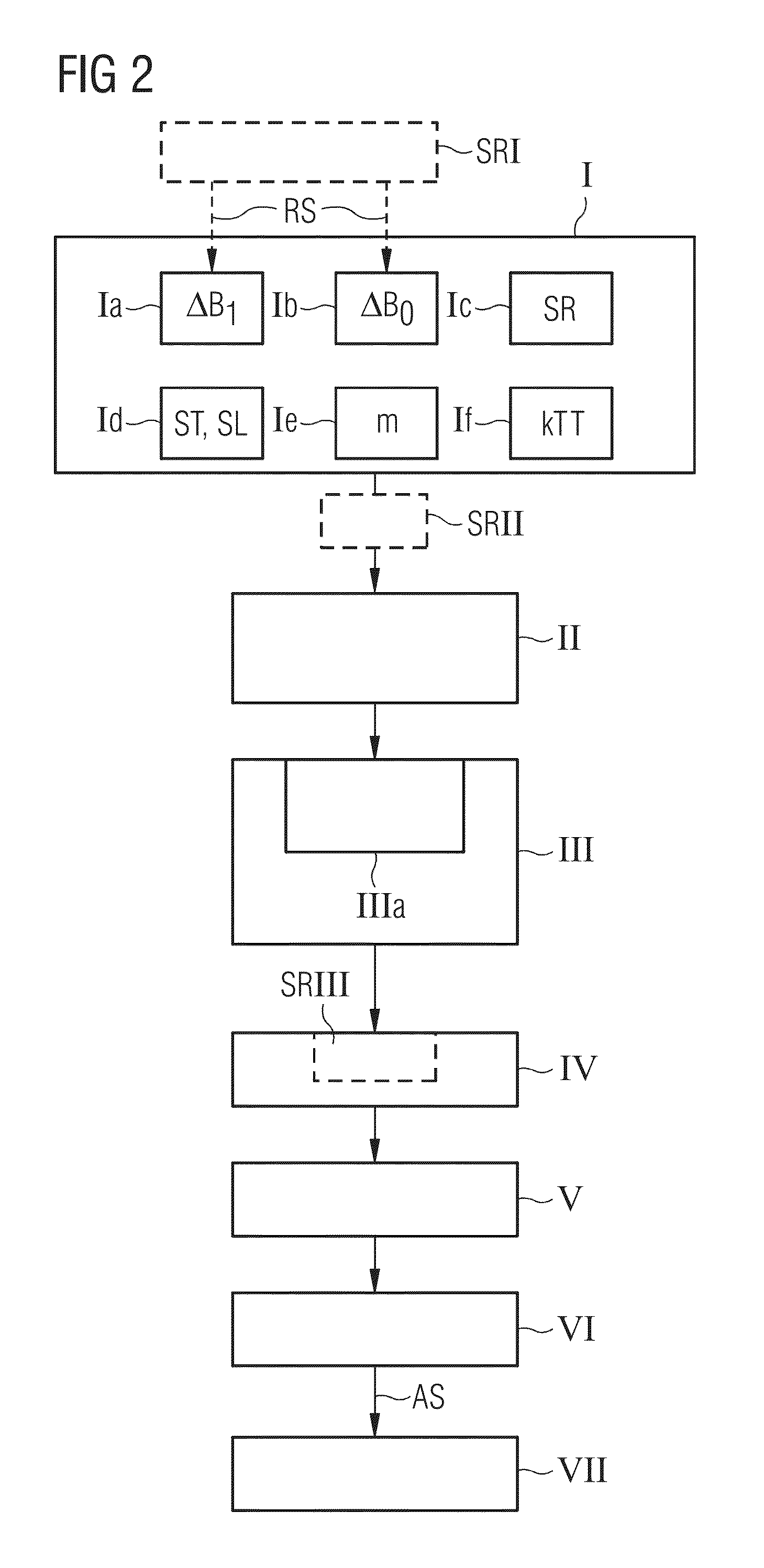
Establishing a Magnetic Resonance System Actuation Sequence
ActiveUS20140292333A1Shorten the construction periodNarrow downMeasurements using NMR imaging systemsElectric/magnetic detectionAcquisition SchemeResonance
A method for establishing a magnetic resonance system actuation sequence is described. A first number of field distribution maps are acquired for slices of the measurement region, and a radiofrequency pulse train is established on the basis thereof for the magnetic resonance system actuation sequence. This acquisition of the first number of field distribution maps may be brought about on the basis of an acquisition scheme. A reduced number of field distribution representation maps are established on the basis of the acquired field distribution maps, which field distribution representation maps represent the first number of acquired field distribution maps in accordance with a predetermined optimization criterion, and the radiofrequency pulse train is established on the basis of the field distribution representation maps.
Owner:SIEMENS HEALTHCARE GMBH
Microbial agent for producing pickles
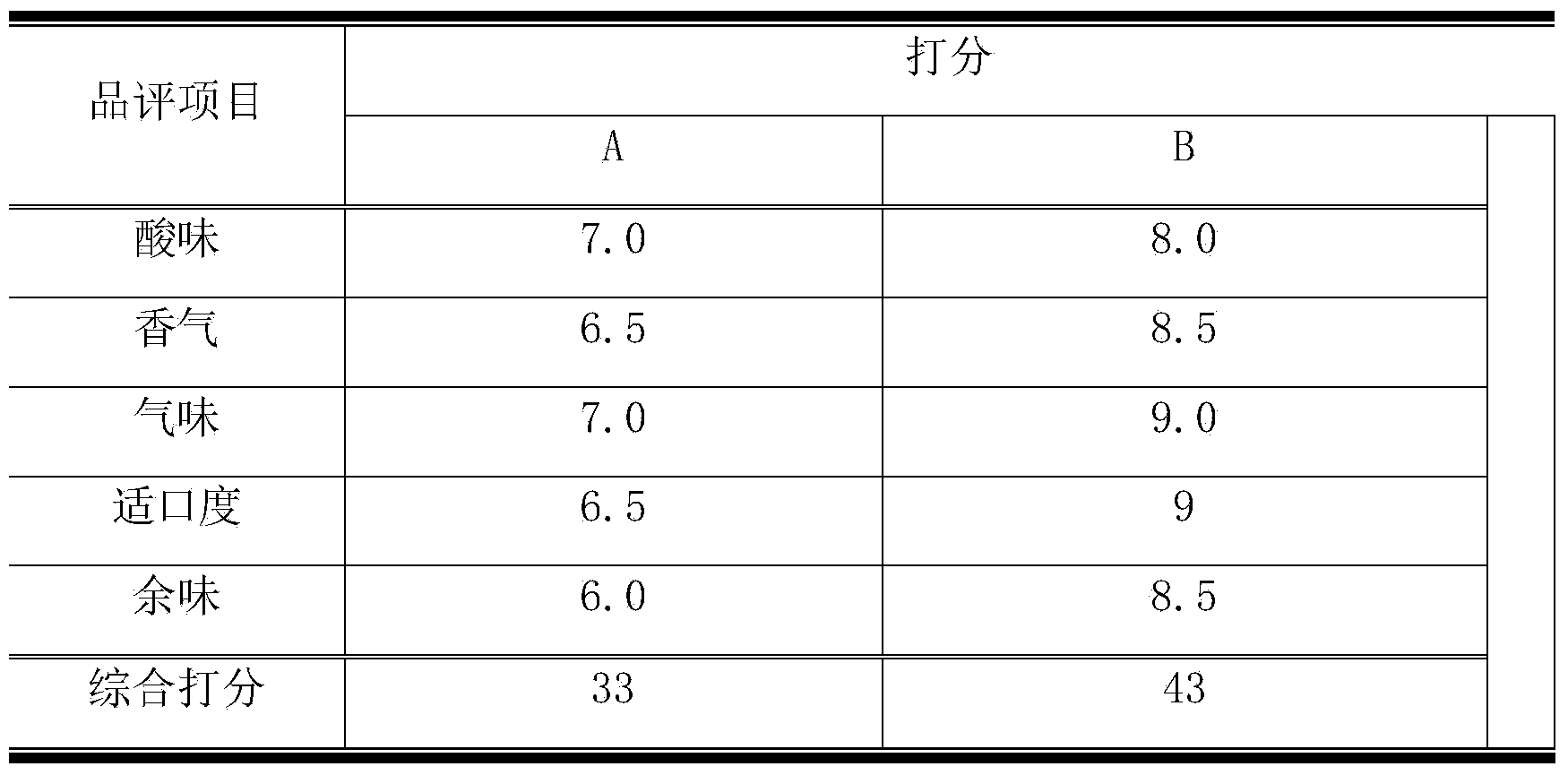
ActiveCN104293706AKeep the flavorSolve the problem of subculture starterFungiBacteriaMicrobial agentMicrobiology
The invention discloses a microbial agent for producing pickles, belonging to the field of production of microbial preparations. The microbial agent product contains lactobacillus plantarum CGMCC NO.9405, and consists of the following agents: lactobacillus plantarum CGMCC NO.9405, bacillus aceticus and saccharomycetes. The preparation method comprises the following steps: singly culturing the lactobacillus plantarum, bacillus aceticus and saccharomyces cerevisiae strains, centrifuging to collect the thallus after culturing by a preset period of time, and preparing microbial powder of each strain by utilizing a conventional microbial preparation production method; and mixing and blending the prepared microbial powder according to a ratio. According to the selection and ratio of the strains, the production speed, good flavor and quality of pickle products are guaranteed, so that the production safety of the pickles is high, and the product is standard and consistent, can be suitable for large-scale industrial production and manual workshop type production and has wide application market.
Owner:拜肯生物科技(上海)股份有限公司
Dental implant and preparation method thereof
ActiveCN102579145AIncrease the binding rateShorten binding timeDental implantsImpression capsMicro arc oxidationTitanium
The invention relates to the field of dental implant materials, and discloses a dental implant carrying miRNA (micro-ribonucleic acid) or siRNA (small interfering ribonucleic acid) and a preparation method thereof. The outer surface of the dental implant is a microarc oxidation coating with micro / nano appearance, wherein factors promoting osteogenic differentiation are loaded on the microarc oxidation coating, and are miRNA or siRNA. The preparation method comprises the following steps: processing pure titanium or titanium alloy into an implant matrix; polishing, cleaning and blow-drying; producing the microarc oxidation coating with micro / nano appearance on the outer surface of the implant matrix by adopting a microarc oxidation process, cleaning and blow-drying; mixing liposome and miRNA or siRNA to produce a carrier mixture; immersing the implant matrix into the carrier mixture, freezing with drikold; and loading the carrier mixture to the microarc oxidation coating by adopting a vacuum lyophilisation process, and standing at normal temperature to obtain the dental implant carrying miRNA or siRNA.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Vegetable fermentation composite bacterial agent product
InactiveCN104312941AKeep the flavorSolve the problem of subculture starterFungiBacteriaBiotechnologyProduction rate
The invention discloses a vegetable fermentation composite bacterial agent product for pickled vegetable production, and belongs to the microbial preparation production field. The vegetable fermentation composite bacterial agent is composed of the following bacterial agents: lactobacillus plantarum CGMCC No.9405, lactobacillus rhamnosus, bacillus aceticus, and yeast. Firstly, lactobacillus plantarum, bacillus aceticus, lactobacillus rhamnosus and saccharomyces cerevisiae strains are cultured individually, are cultured for a predetermined time and then are centrifuged to collecting thalli, and powdered microbial powders of the strains are prepared by using a conventional microbial preparation production method; the prepared stain powders are mixed and blended according to the proportion, wherein the composition ratio of the stains is also obtained through carefully experimental investigation; selection and proportioning of the strains guarantee the production rate of pickled vegetable products, excellent flavor of the pickled vegetable products and good quality of the pickled vegetable products.
Owner:宁波北仑锐晟明杰生物科技发展有限公司
Integrated implant system (IIS) biocompatible, biodegradable and bioactive, comprising a biocompatible sterile porous polymeric matrix and a gel, integrating in situ the tridimensional matrix structure
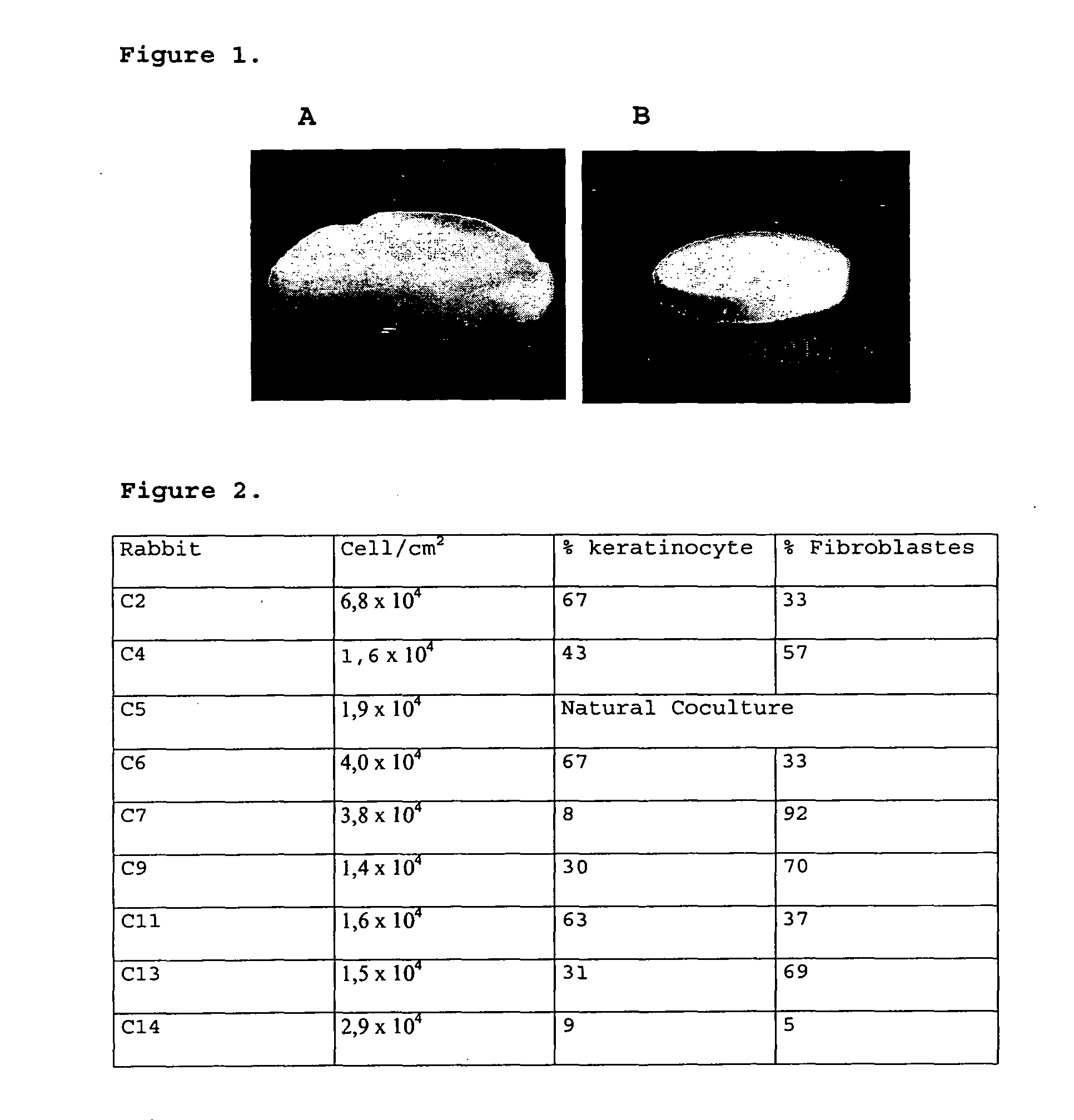
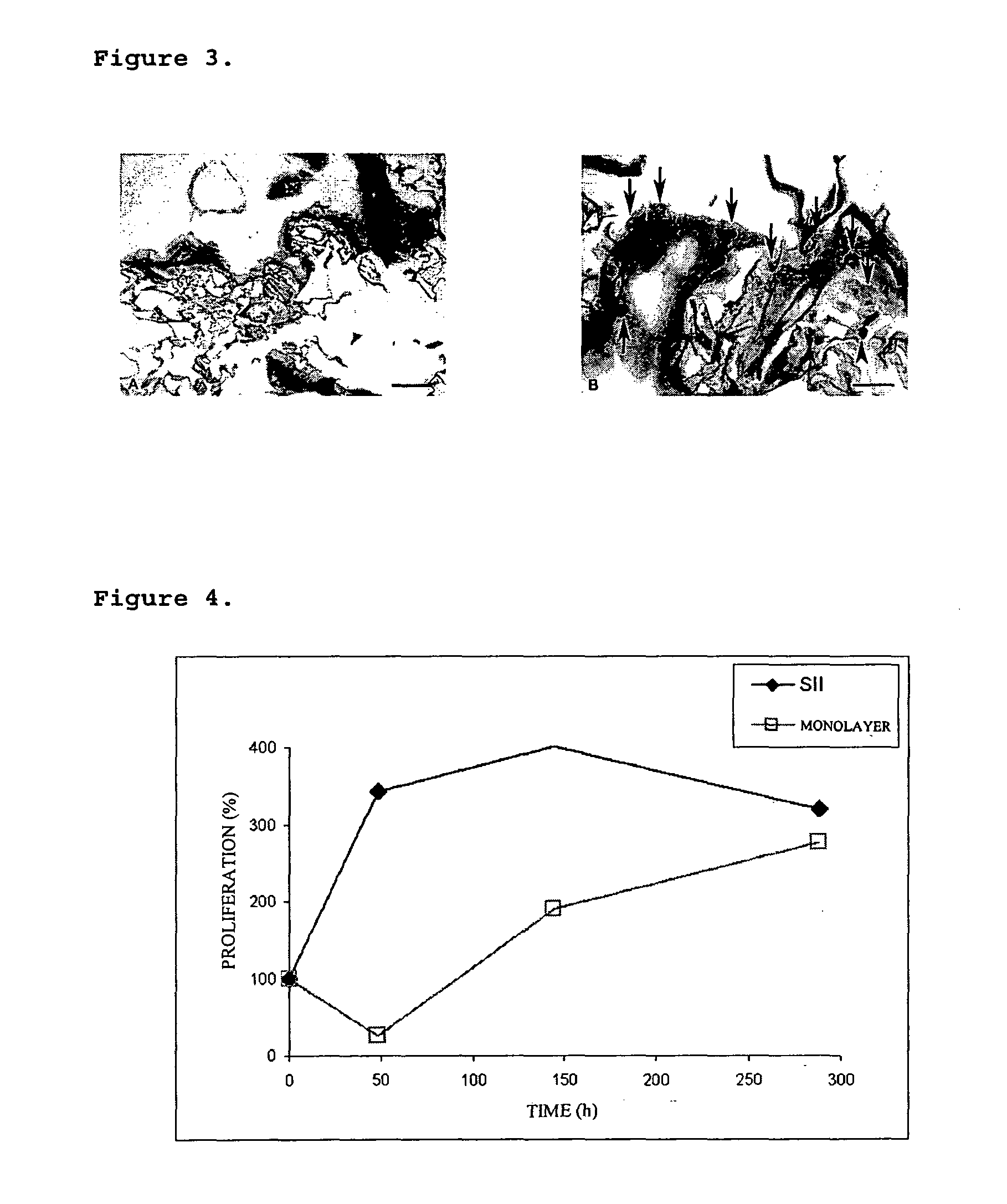
The present invention refers to an Integrated Implant System constituted as a gel-matrix-cells integrated system, that allows providing implants in a brief time period, for covering a great skin extension to be treated, with a successful acceptance in patients with burns, chronic damage or wound skin, needing of a skin grafting.
Owner:UNIV TECNICA FEDERICO SANTA MARIA +2
Automatic production system for inductance coil
InactiveCN102930976AIncrease productivityReduce manufacturing costCoils manufactureWinding machineSpot welding
The invention discloses an automatic production system for an inductance coil. The automatic production system comprises a four-axis winding machine, an automatic feeding bending machine, an automatic feeding upward-downward laser paint stripping machine, an automatic feeding electrode plate charging machine and an automatic feeding cover upper cover plate resistance spot welding machine, which are sequentially connected through a tool conveyor belt. According to the automatic production system for the inductance coil, all procedures for manufacturing the inductance coil can be automatically finished, production efficiency is improved, and production cost is lowered.
Owner:SHIN YUAN ELECTRONICS PROD KUNSHAN
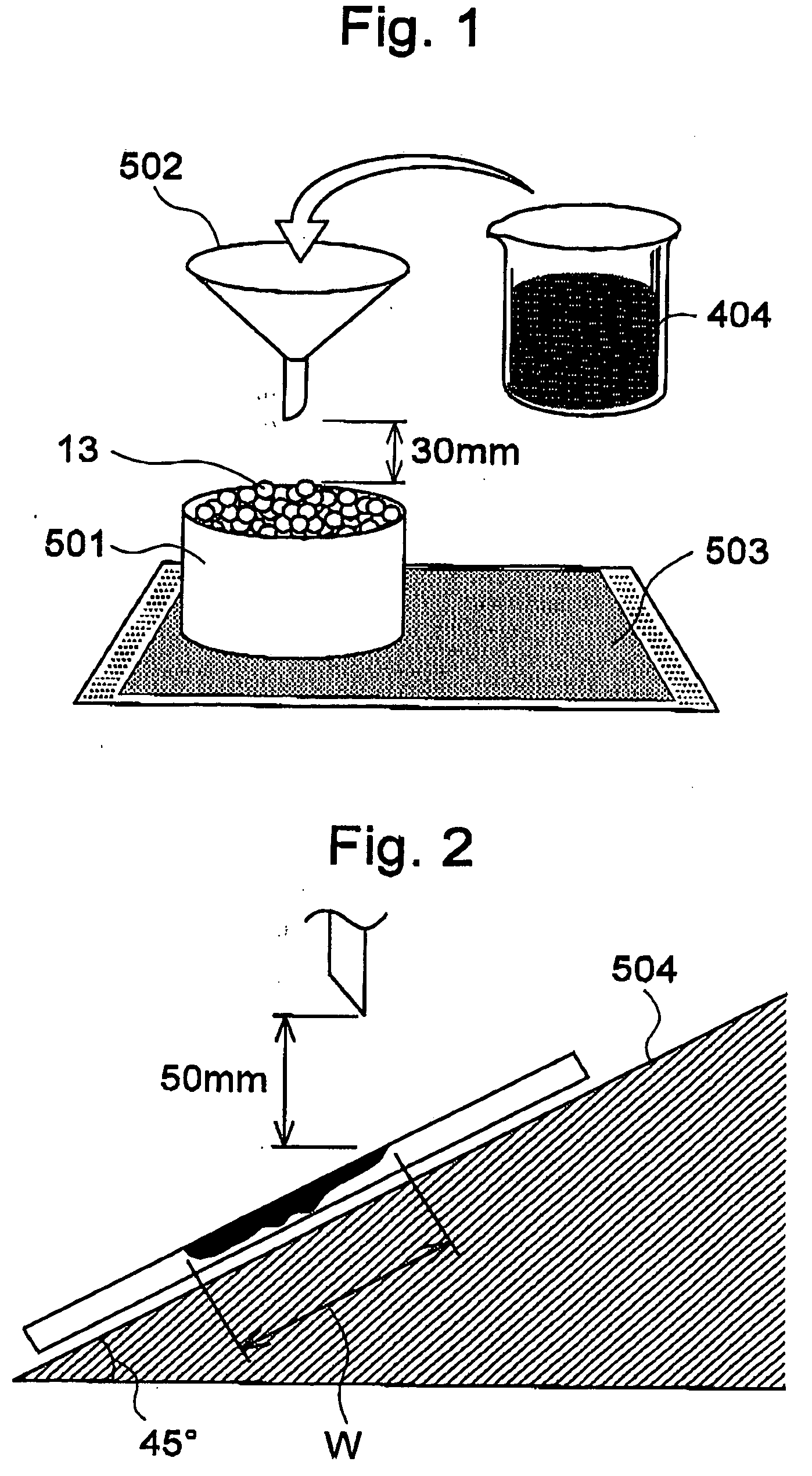
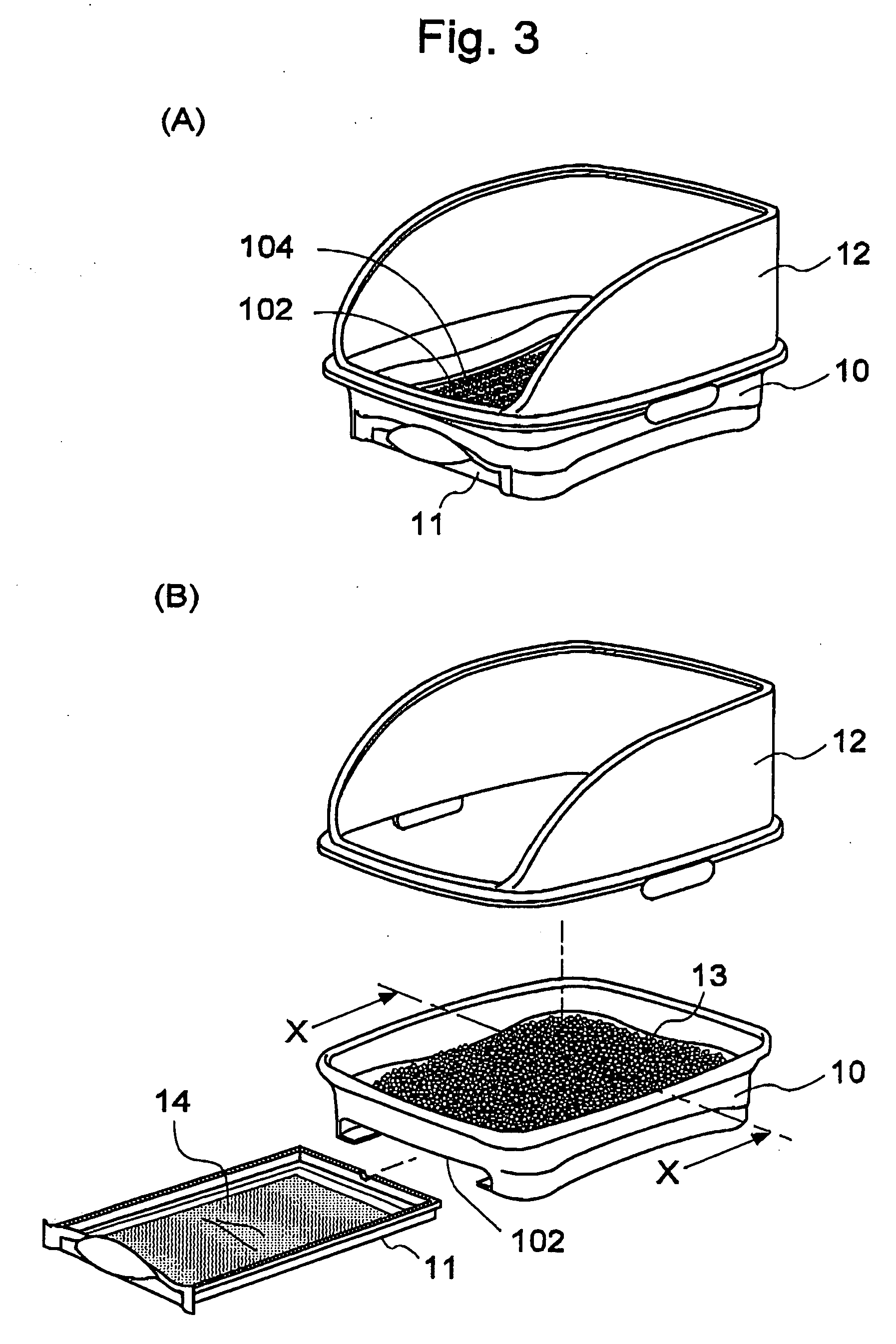
Animal litter
ActiveUS20060112894A1Absorption speed can be suppressedDry stateAnimal housingOther apparatusAnimal scienceGranular material
Provided is a litter, related to litter used for animal litter, which suppresses odors due to excretory substances remaining in litter and litter container using thereof. This animal litter comprises a plurality of granular materials, one part of a surface thereof comprising first granular material having water-shedding properties and second granular material having liquid-absorbing properties.
Owner:UNI CHARM CORP
Flower and fruit-flavored brown sugar solid drink and preparation method thereof
InactiveCN106261329AGood molding effectAvoid deformationNatural extract food ingredientsFood ingredient as mouthfeel improving agentUltrasonic assistedFruit juice
The invention discloses flower and fruit-flavored brown sugar solid drink and a preparation method thereof. The brown sugar solid drink comprises the following components in parts by weight: 94-97 parts of brown sugar and 3-6 parts of a compound extract juice, wherein the compound extract juice consists of medlar juice, longan juice, red jujube juice and rose juice; the medlar juice, the longan juice, the red jujube juice and the rose juice are prepared through ultrasonic-assisted extraction. According to the brown sugar solid drink, flower and fruit juice is extracted through ultrasonic-assisted extraction, so that the brown sugar solid drink has the advantages of high nutrient leaching rate, time conservation, energy conservation and high efficiency; meanwhile, no additive is added, so that the nutritional value and the mouth feel of the drink are enhanced.
Owner:赖维新
Dendritic macromolecule catalytic hair dyeing agent and preparation and use methods thereof
InactiveCN110123670ANot easily oxidizedGood saveCosmetic preparationsHair cosmeticsMiddle medullaPhosphoric acid
The invention discloses a dendritic macromolecule catalytic hair dyeing agent and preparation and use methods thereof, wherein the dendritic macromolecule catalytic hair dyeing agent comprises an agent A and an agent B. The agent A consists of the following components: 5,6-dihydroxy indole, cetostearyl alcohol, oleic acid, propylene glycol, isopropanol, sodium dodecyl sulfonate, a polyethylenediamine dendritic polymer, ammonia water and water. The agent B consists of the following components: hydrogen peroxide, cetostearyl alcohol, oleic acid, propylene glycol, isopropanol, sodium dodecyl sulfonate, phosphoric acid and the like. The preparation method comprises the steps: firstly, preparing the agent A, and then preparing the agent B. The use method comprises the steps: mixing the agent Aand the agent B in a proportion of 1:1, stirring evenly, then applying to hair, and 30 min later, cleaning the hair. The 5,6-dihydroxyindole in the hair dyeing agent is not easy to oxidize and is easyto preserve in air, has quite obvious dyeing effect is and can enter the interior of hair medulla of hair and the hair dyeing agent is washed and is not easy to fade after dyeing.
Owner:SICHUAN UNIV
4-methoxy methyl acetoacetate preparation method
ActiveCN104478719AReduce usageReduce the introductionOrganic compound preparationCarboxylic acid esters separation/purificationDistillationInternal temperature
The invention discloses a 4-methoxy methyl acetoacetate preparation method. The 4-methoxy methyl acetoacetate preparation method comprises the following steps of adding a solvent tetrahydrofuran into a reaction kettle and leading inert gas into the reaction kettle, setting the internal temperature of the reaction kettle to be 15 DEG C to 25 DEG C, adding industrial sodium hydride and a metal alkaline compound in a stirring state, then adding the solvent tetrahydrofuran, dropwise adding mixed liquid of methyl alcohol and methyl-4-chloroacetoacetate at the temperature of lower than 20 DEG C to perform reaction for 4-6 hours, rising the temperature to be 20 DEG C to 25 DEG C to continue to react for 4-15 hours, reducing the system temperature to be 6 DEG C to 10 DEG C after TLC detection reaction is completed, adding a hydrochloric acid solution with molar concentration of 2 mol / L to regulate the pH of a system to be 5 to 7, performing standing and laying, concentrating and spin-drying upper-layer liquid to remove the solvent tetrahydrofuran after liquid separation and then obtaining a colorless product 4-methoxy methyl acetoacetate through wiped-film molecular distillation. The 4-methoxy methyl acetoacetate can react at room temperature, a product can be produced at low temperature through distillation, and dangerousness in the production process and product impurity content can be directly and effectively reduced.
Owner:ZHONGSHAN NIKEMEI PHARMA
Sperm DNA fragmentation detection kit
ActiveCN104073555ASimple structureEasy to operateMicrobiological testing/measurementPreparing sample for investigationDiffusionMaterial resources
The invention provides a sperm DNA fragmentation detection kit. The kit comprises an enveloped glass slide, a fusible gel, an A solution, a B solution, a Wright stain, a Wright buffer solution and a SCD (sperm chromatin diffusion) storage solution. The invention further provides a method for detecting sperm DNA fragmentation by using above kit. The reagent structure is optimized according to the detection method so that the reagent is stable and reliable; the operation of detecting by using the reagent is simple and free from special detection instruments, the clinical conventional application and popularization are facilitated; by adopting the unique SCD storage solution, the sperm sample can be effectively stored, the DNA fragmentation rate cannot be changed after being cryopreserved at -20 DEG.C within two weeks, the required sample can be easily stored through the SCD storage solution, the detection can be performed without the limitation of time and number, and large number of manpower and material resources are saved.
Owner:BRED LIFE SCI TECH
SERUM/PLASMA MicroRNAs AND USES THEREOF
ActiveUS20140121133A1Large spectrumHigh sensitivityNucleotide librariesMicrobiological testing/measurementNervous systemTherapeutic effect
This invention provides a combination of microRNAs for evaluating the physiological and / or pathological condition of a subject, wherein the combination comprises all detectable microRNAs stably existing in the serum / plasma of a subject; and a method for evaluating the physiological and / or pathological condition of a subject, wherein the method includes determining all detectable microRNAs stably existing in the serum / plasma of a subject; and a kit for evaluating the physiological and / or pathological condition of a subject, wherein the kit contains the tools for determining all detectable microRNAs that stably existing in the serum / plasma of a subject; and a biochip for evaluating the physiological and / or pathological condition of a subject, wherein the biochip contains the components for determining all detectable microRNAs stably existing in the serum / plasma of a subject. The aforementioned combination, method, kit and biochip can be used for diagnosis as well as differentially diagnosis of diseases including various tumors; various acute / chronic infectious diseases, e.g. viral diseases such as viral influenza, viral hepatitis, AIDS, SARS, bacterial diseases such as tuberculosis, bacterial pneumonia, and other acute / chronic infectious diseases caused by various pathogenic microorganisms; other acute / chronic diseases such as diseases of respiratory system, diseases of immune system, diseases of blood and hematopoietic system, diseases of circulatory system such as cardio-cerebrovascular diseases, metabolic diseases of endocrine system, diseases of digestive system, diseases of nervous system, diseases of urinary system, diseases of reproductive system and diseases of locomotor system, prediction of complications occurrence and malignant diseases relapse, evaluation of therapeutic effects, screening of pharmaceutical active ingredients, assessment of drug efficacy as well as forensic authentication and prohibited drug inspection and the like, possessing a number of advantages such as extensive detection spectrum, high sensitivity, low cost, convenience for sampling, ease for sample preservation, etc. The said method can be widely used in work related to general survey of diseases and so on, improve the low-specificity and low-sensitivity caused by individual differences which single markers are difficult to overcome, significantly increasing the clinical detection rate of diseases, all of which make it become an effective means for diagnosing diseases in an early phase.
Owner:JIANGSU MINGMA BIOTECH
Gas-filled protective display casing
The invention relates to an aerated protective show frame which can store and show calligraphy and painting and cultural relics for ever, wherein the invention is characterized in that: putting matters into frame 1; putting frame 1 into pressure-resistant chamber 3; vacuuming the chamber 3; filling pure inert gas, to make show frame without oxygen, dust, steam, harmful air, microbe and mold; therefore, even the condition changes, the pressure will be same as atmosphere pressure, to avoid harmful physical, chemical and biological functions, to store and show matter with low cost.
Owner:陈家山
Hygienic agent for use in hemodialysis
InactiveUS6162394AEasy to useReduce contact timeBiocideOther blood circulation devicesAcetic acidHaemodialysis machine
The invention concerns a hygienic agent for use in hemodialysis. This hygienic agent based on peracetic acid has an aqueous solution containing 6 to 8 weight % hydrogen peroxide, 0.1 to 1 weight % peracetic acid and 2 to 10 weight % acetic acid. Application to the disinfection, sterilization of dialysis generators, dialyzers, hemofilters and hemodialyzers and circuits of water treatment for hemodialysis.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Flat emitter
InactiveUS9530603B1Good saveDistanceX-ray tube electrodesDischarge tube solid thermionic cathodesEffect lightEngineering
A flat emitter comprises four current-supply heating legs. Half lighting for a small focus in which a current is supplied to heat only a region narrower and full lighting for a large focus in which a current is supplied to heat the entire region are selectable according to the combination of the legs. Either one of a set of the two full-lighting current-supply heating legs for the full lighting and a set of the two half-lighting current-supply heating legs for the half lighting is linearly formed, and the other is formed to be bent plural times in zigzag to set the space between the full-lighting current-supply heating leg and the half-lighting current-supply heating leg, which are adjacent to each other, at their terminals to be larger than the space at their base parts.
Owner:SHIMADZU CORP
Tumor vaccine using salmonella as carrier and preparation method thereof
InactiveCN101204584AImproving immunogenicityAntigen expression is large and stableBacteriaGenetic material ingredientsConfusionType three secretion system
The invention discloses a tumor vaccine using attenuated salmonella as a vector and a preparation method thereof.Active component of the tumor vaccine is recombinant attenuated salmonella, the construction method of the recombinant attenuated salmonella includes the following steps: 1) an expression vector for expressing recombinant prokaryotic cell of confusion protein is constructed; the confusion protein comprises a N side signal area (namely a N side guiding secretion area[1]) of effect proteins(SPI-2 TTSS) in a III-type secretion system of salmonella and at least a protein antigen or a protein epitope of the tumor; the expression vector of the recombinant prokaryotic cell is obtained by introducing encoding gene of the confusion protein into multiple cloning sites of the expression vector of the prokaryotic cell; 2) the expression vector of the recombinant prokaryotic cell is introduced to the attenuated salmonella to obtain the recombinant attenuated salmonella expressing the confusion protein. The tumor vaccine in the invention can be used for preventing the tumor.
Owner:BEIJING NACK CHANGJIN INT ARGENTUM ION TECH DEV
Sample plate for MALDI mass spectrometry and process for manufacture of the same
ActiveUS7619215B2Good adhesionEasy detachmentSamplingSamples introduction/extractionCost effectivenessMass Spectrometry-Mass Spectrometry
The present invention relates to a sample microfocusing plate useful in MALDI mass spectrometry having a patterned hydrophobic organosilane coating layer and at least a central portion formed on the surface and a process for manufacturing and using the sample microfocusing plate. The sample microfocusing plate can rapidly dry the solvent contained in samples leading to efficient sample analysis, and can be prepared by cost effectiveness.
Owner:ASTA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com