Serum/plasma micronas and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
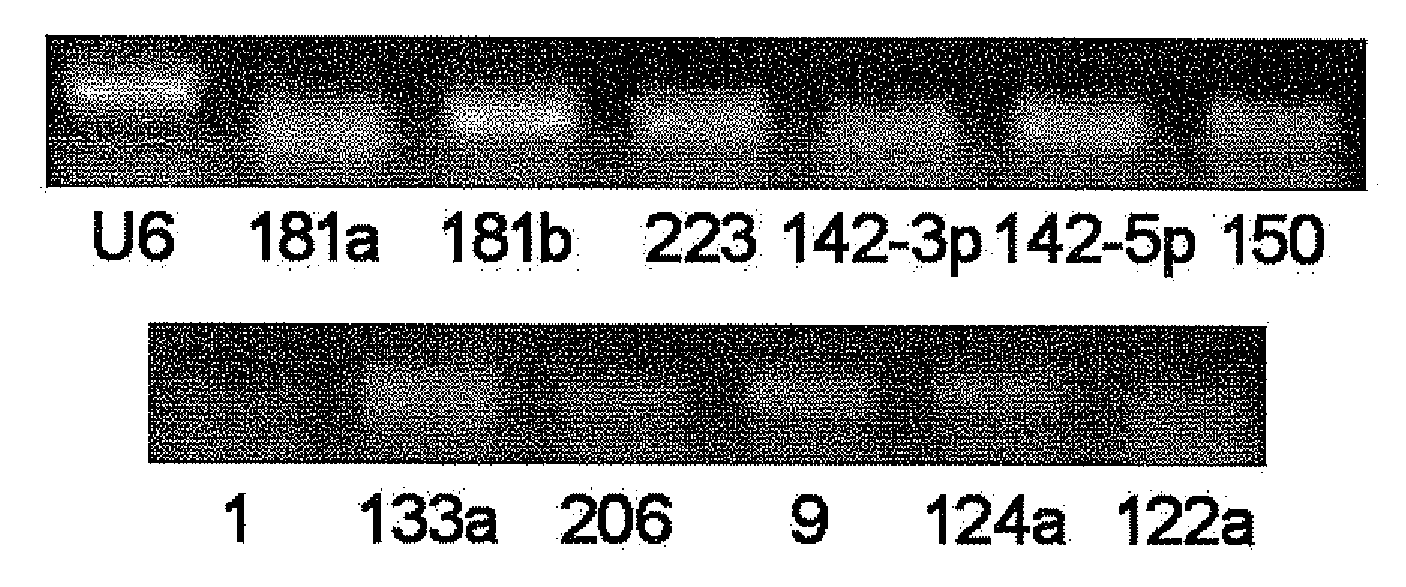
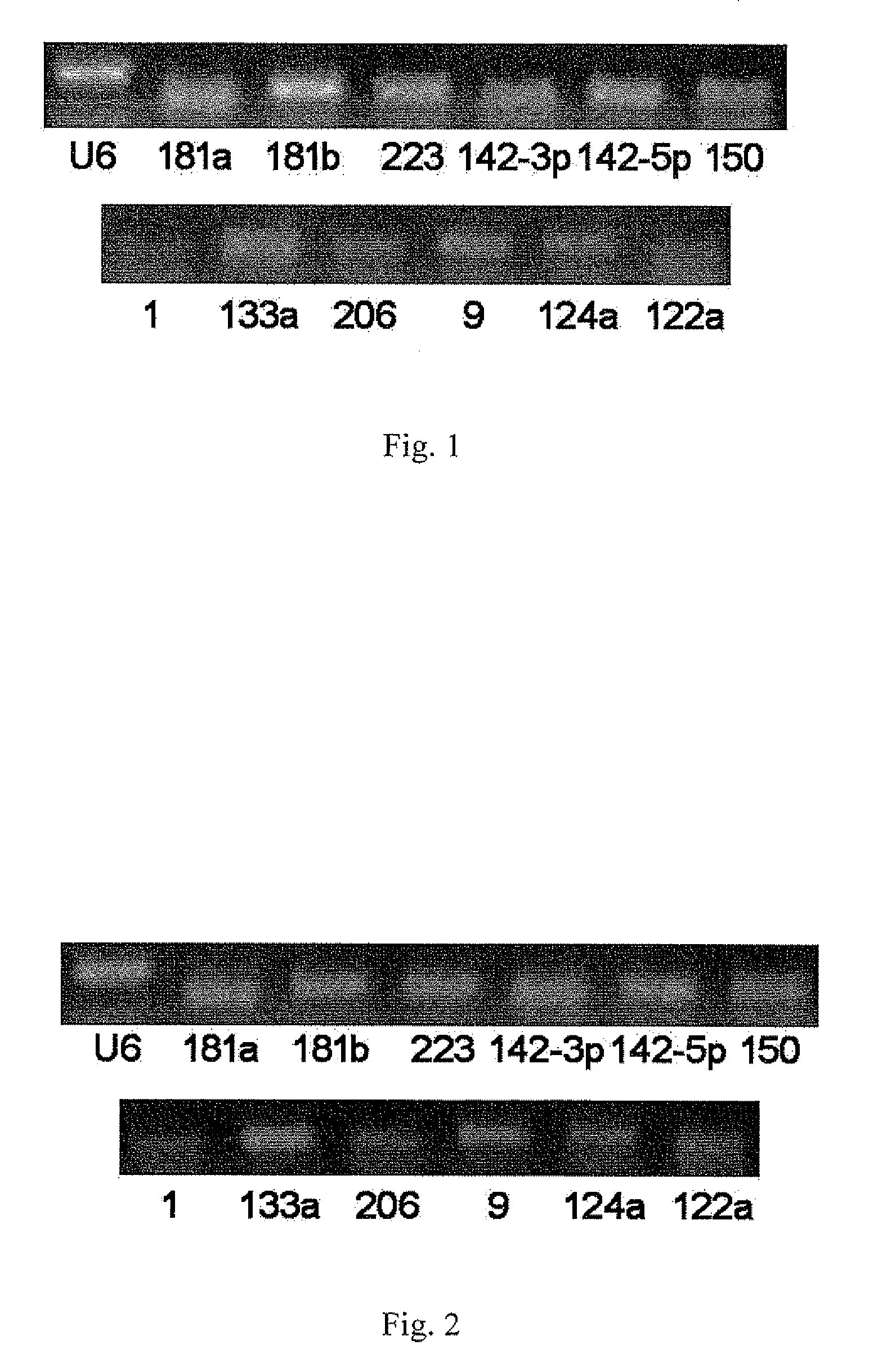
[0051]The RT-PCR Experiments of MicroRNAs in Serum / Plasma
[0052]By using RT-PCR technique, it is found and proved that there stably exist various microRNAs in serum / plasma of both human beings and animals, and that their expression levels are considerably high. The specific RT-PCR steps are as follows:
[0053](1) collecting serum / plasma of mice, rats, normal persons and some patients;
[0054](2) preparing samples of cDNA. This operation has two options: one is to directly conduct reverse transcription reaction using 10 μl of serum / plasma; the other is to firstly extract the total RNA from serum / plasma (usually, about 10 μg of RNA can be enriched from 10 ml of serum / plasma) with Trizol reagent (Invitrogen Co.), subsequently obtain cDNA through RNA reverse transcription reaction. The reaction system of reverse transcription includes 4 μl 5×AMV buffer, 2 μl 10 mM each dNTP mixture (Takara Co.), 0.5 μl RNase Inhibitor (Takara Co.), 2 μl AMY (Takara Co.) and 1.5 μl gene specific reverse prime...
example 2
The Real-Time PCR Experiments of MicroRNAs in Serum / Plasma
[0058]Quantitative PCR experiments of microRNAs in serum / plasma are conducted to study the specific variation of microRNAs quantity in serum / plasma during the course of various diseases, including various tumors, various acute and chronic infectious diseases, e.g. viral diseases such as viral influenza, viral hepatitis, AIDS, SARS, bacterial diseases such as tuberculosis, bacterial pneumonia, and other acute and chronic infectious diseases caused by various pathogenic microorganisms; other acute and chronic diseases such as diseases of respiratory system, diseases of immune system, diseases of blood and hematopoietic system, diseases of circulatory system such as cardio-cerebrovascular disease, metabolic diseases of endocrine system, diseases of digestive system, diseases of nervous system, diseases of urinary system, diseases of reproductive system and diseases of locomotor system. The experimental principles and experimenta...
example 3
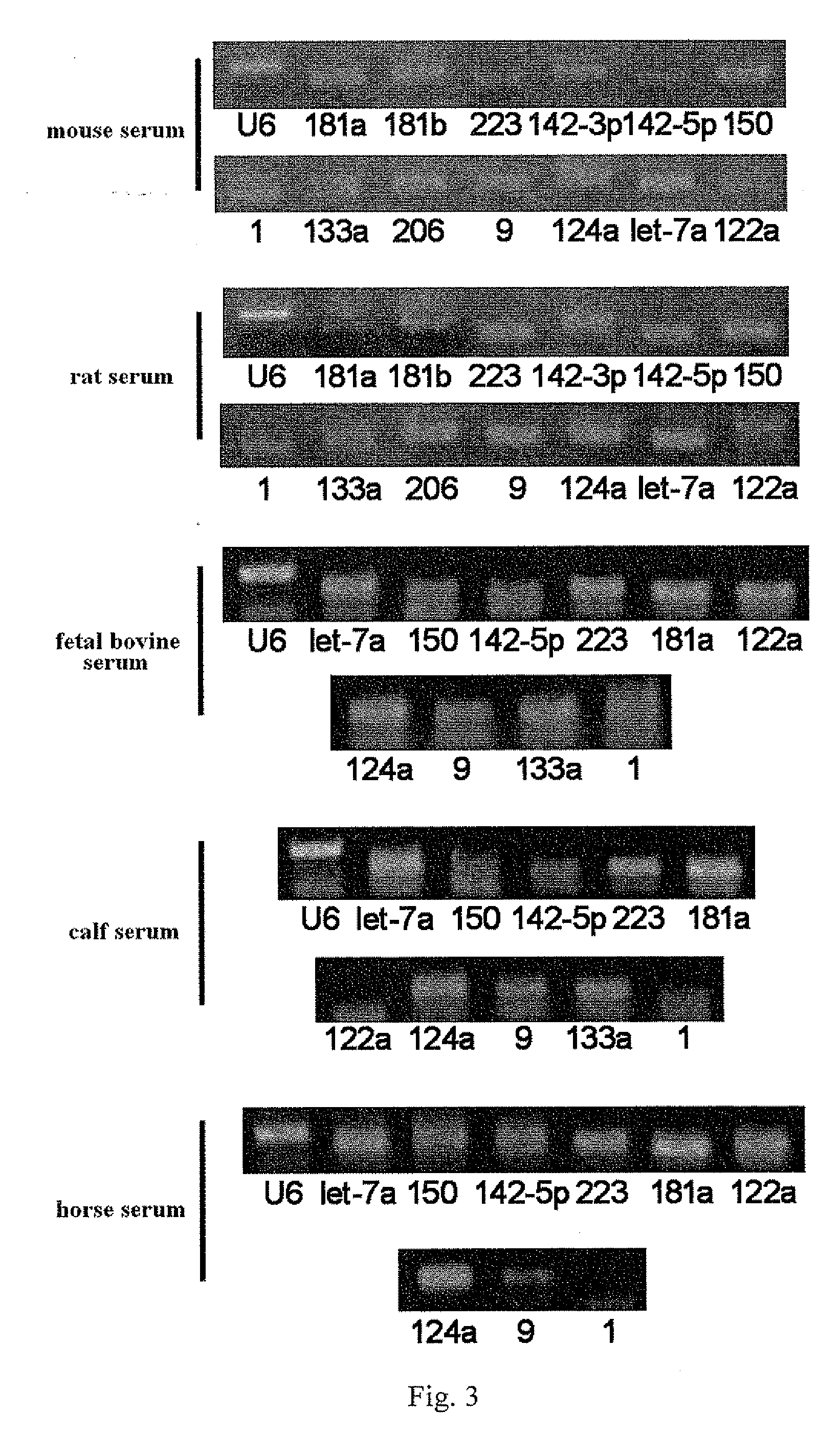
The Superiority of Serum / Plasma MicroRNAs as Disease Markers
[0060]Through detecting the quantities of microRNAs and macroRNAs in serum and blood cells, it is found that there is an abundant content of microRNAs in serum. See FIG. 5. As represented by U6 molecules with a molecular weight of 100 bp and ribosomal RNA molecules with molecular weights being 18S and 28S respectively, the quantity of macroRNAs in blood cells is at least tens times that in serum; while the quantity of microRNAs in serum remains the same as that in blood cells except the microRNAs with blood cell specificity. Therefore, serum / plasma will specifically enrich small molecule RNAs, especially microRNAs.
[0061]It is also found that microRNAs are to some extent able to resist the action of endonuclease, which is possibly one of the reasons why microRNAs can stably exist in serum / plasma. Total RNAs extracted from cultured cell line are processed with endonuclease RNase A and the remaining quantity of macroRNAs and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com