Environmentally-friendly synthetic method of pyrimidine compound
A synthesis method and compound technology, which is applied in the synthesis field of pyrimidine compounds, can solve problems such as difficult recovery of catalysts, unfriendly solvent environment, and strong corrosion of equipment, so as to reduce pollution and corrosion of equipment, and the product yield is not affected. Effect, yield and effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
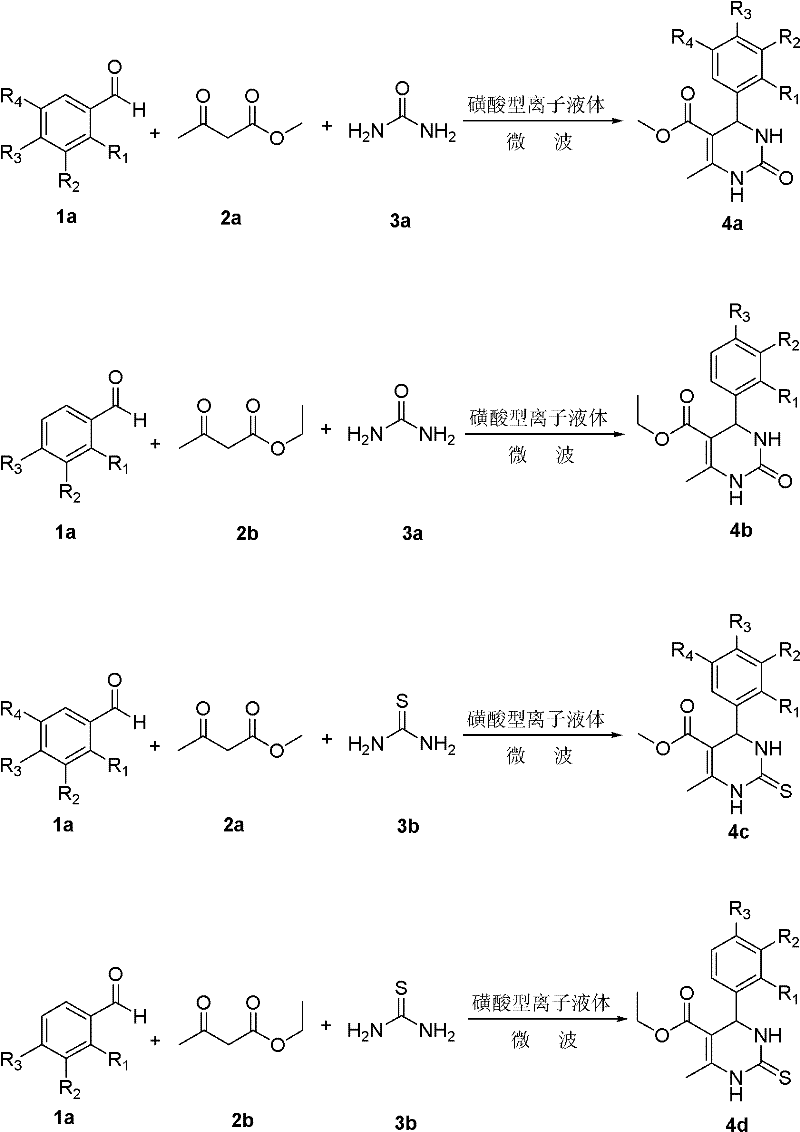
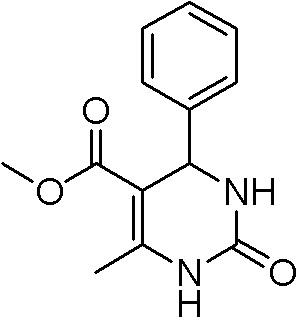
[0027] Sulfonic acid type ionic liquid [(HSO 3 -p) 2 im][CF 3 SO 3 ] (0.02mmol) was added into a three-necked round-bottomed flask, benzaldehyde (25mmol), methyl acetoacetate (27.5mmol) and urea (37.5mmol) were added to the reaction vessel successively, and under mechanical stirring, placed in microwave reaction In the container, react with microwave at 100°C for 10 minutes, cool to room temperature, pour the reaction solution into 30 mL of ice water and stir for 20 minutes, filter the mixed solution directly, and dry it. The yield is 97%. The structural formula is as follows:
[0028]
Embodiment 2
[0030] Sulfonic acid type ionic liquid [(HSO 3 -p) 2 im][CF 3 SO 3 ] (0.02mmol) joins in the three-necked round bottom flask, benzaldehyde (25mmol),
[0031] Ethyl acetoacetate (27.5mmol) and urea (37.5mmol) were successively added to the reaction vessel, placed in a microwave reactor under mechanical stirring, and microwaved at 100°C for 10 minutes, cooled to room temperature, and the reaction solution was poured into Stir in 30mL of ice water for 20 minutes, filter the mixture directly, and dry it with a yield of 98%. The structural formula is as follows:
[0032]
Embodiment 3
[0034] Sulfonic acid type ionic liquid [(HSO 3 -p) 2 im][CF 3 SO 3 ] (0.02mmol) joins in the three-necked round bottom flask, benzaldehyde (25mmol),
[0035] Methyl acetoacetate (27.5mmol) and thiourea (37.5mmol) were successively added to the reaction vessel, placed in a microwave reactor under mechanical stirring, and microwaved at 100°C for 15 minutes, cooled to room temperature, and the reaction solution poured Pour into 30mL of ice water and stir for 20 minutes, the mixture is directly filtered and dried with a yield of 90%. The structural formula is as follows:
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com