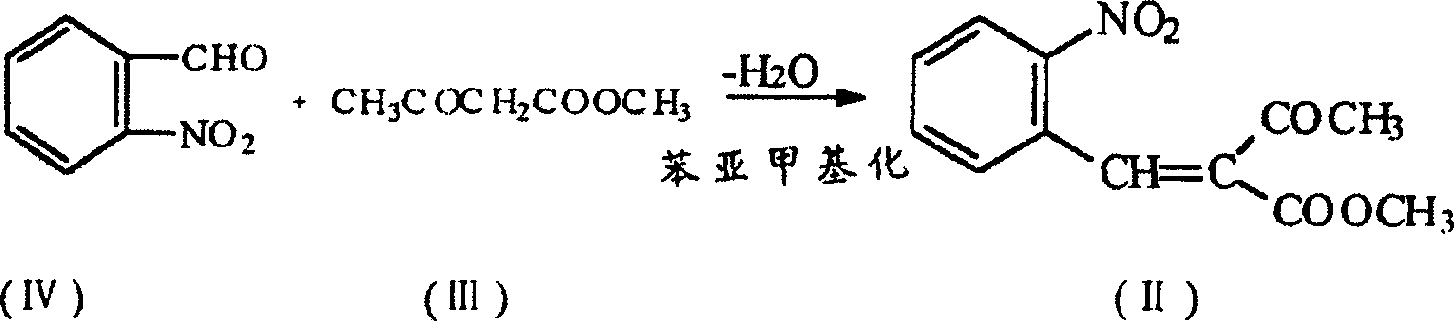
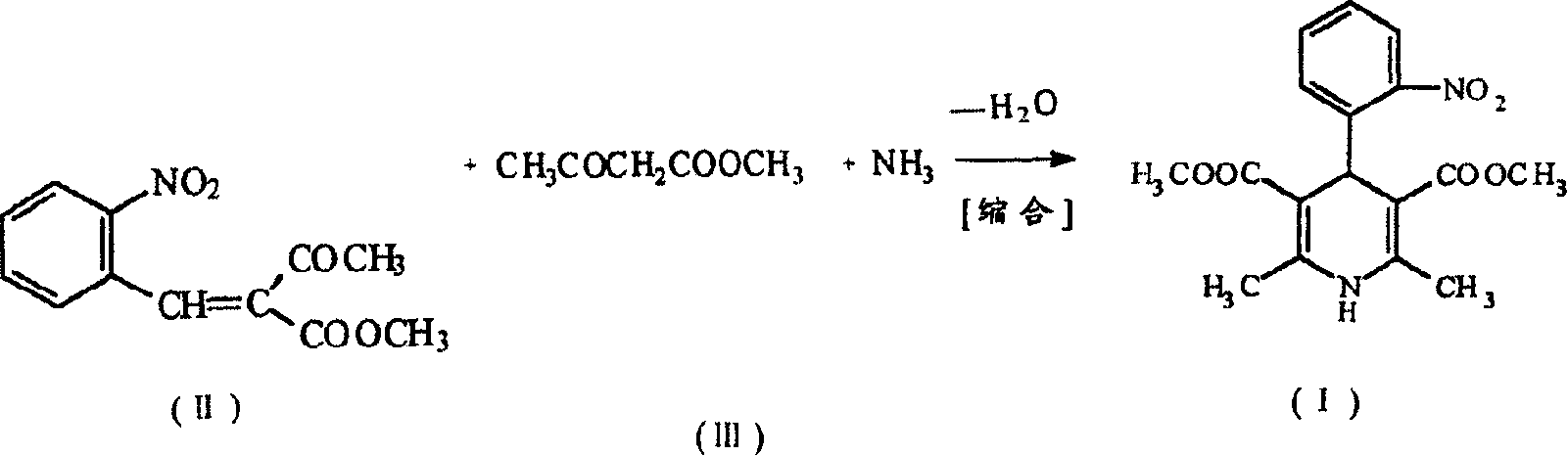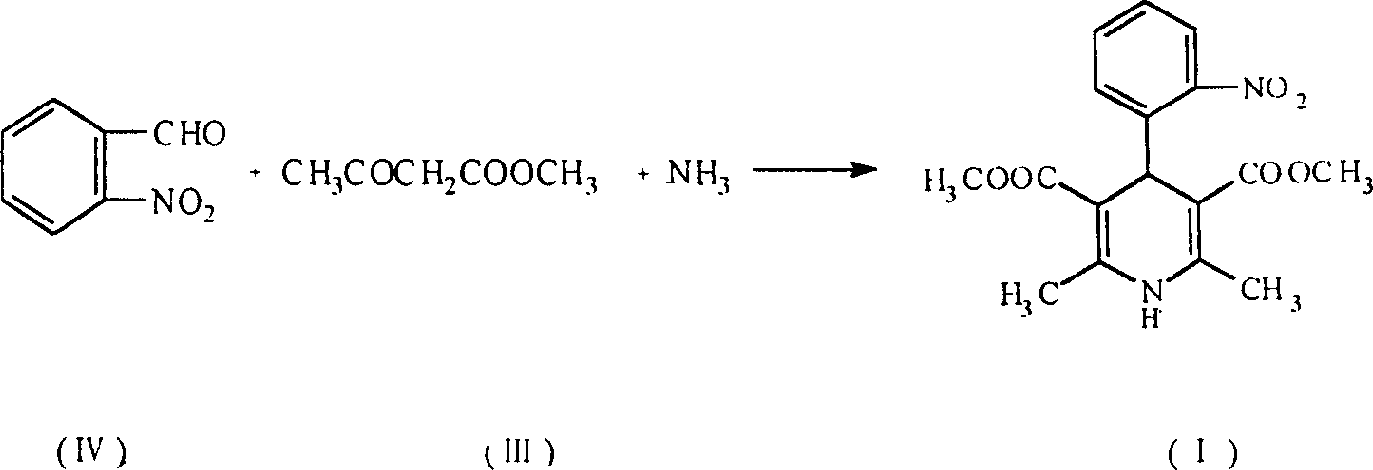Prepn. process of nifedipine
A technology of nifedipine and o-nitrobenzaldehyde, applied in the direction of organic chemistry, can solve the problems of many by-products, low yield, cumbersome reaction process, etc., and achieve the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: in the flask that stirrer is equipped with, add the pyridine carboxylate of o-nitrobenzaldehyde 35g (0.23mol), methyl acetoacetate 28g (0.24mol), ethanol 56ml and catalytic amount, stir at 60 ℃ After reacting for 1 hour, a pale yellow benzylidene solution was obtained.
[0027] Add methyl acetoacetate and 21 ml of ammonia water to the above reaction solution, heat up and reflux for 7 hours, and filter to obtain 70 g of wet weight of crude nifedipine. Without drying the crude product, add 5 times the amount (V / W) of ethanol and 1g of activated carbon, heat and boil for 15 minutes, filter while hot, wash the carbon layer with 20ml of hot ethanol, cool the filtrate to 5°C, filter and wash the wet product at 60 Dry at ℃ to get 57g of nifedipine, mp172℃~173℃, yield 71.1%
Embodiment 2
[0028] Embodiment 2: in the flask that stirrer is equipped with, add the pyridine carboxylate of o-nitrobenzaldehyde 35g (0.23mol), methyl acetoacetate 28g (0.24mol), acetonitrile 35ml and catalytic amount, at 50 ℃ The reaction was stirred for 2 hours to obtain a pale yellow benzylidene solution.
[0029] Add methyl acetoacetate and 21ml of ammonia water to the above reaction solution, raise the temperature, react at 85°C for 5 hours, stir and cool down to 5°C, and filter to obtain 62g wet weight of crude nifedipine. Without drying the crude product, add 5 times the amount (V / W) of ethanol and 1g of activated carbon, heat and boil for 15 minutes, filter while hot, wash the carbon layer with 20ml of hot ethanol, cool the filtrate to 5°C, filter and wash the wet product at 60 After drying at ℃, 52.3g of nifedipine was obtained, mp 172℃~173℃, yield 65.2%.
Embodiment 3
[0030] Embodiment 3: in the flask that stirrer is equipped with, add the pyridine carboxylate of o-nitrobenzaldehyde 35g (0.23mol), methyl acetoacetate 28g (0.24mol), methyl alcohol 58ml and catalytic amount, stir at 50 ℃ After reacting for 2 hours, a pale yellow benzylidene solution was obtained.
[0031] Add methyl acetoacetate and 21 ml of ammonia water to the above reaction solution, heat up and reflux for 7 hours, stir and cool down to 5° C., and filter to obtain 70.5 g of wet weight of crude nifedipine. Without drying the crude product, add 5 times the amount (V / W) of ethanol and 1g of activated carbon, heat and boil for 15 minutes, filter while hot, wash the carbon layer with 20ml of hot ethanol, cool the filtrate to 5°C, filter and wash the wet product at 60 After drying at ℃, 57.1 g of nifedipine was obtained, mp 172℃~173℃, yield 71.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com