Patents
Literature
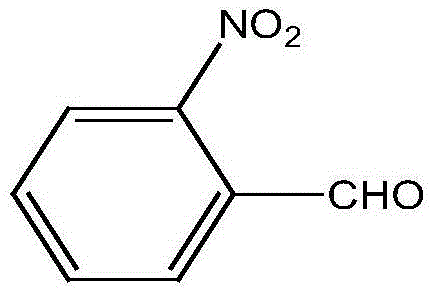
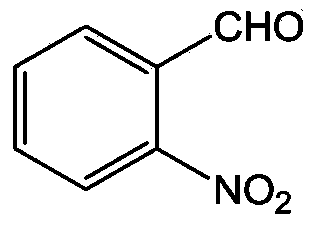
90 results about "O-nitrobenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bis-Schiff-base-connected symmetrical phenanthroimidazole Fe<3+> fluorescent probe and preparation method thereof
InactiveCN105694866AHigh selectivityResponsiveOrganic chemistryFluorescence/phosphorescenceOrganic solventFluorescence
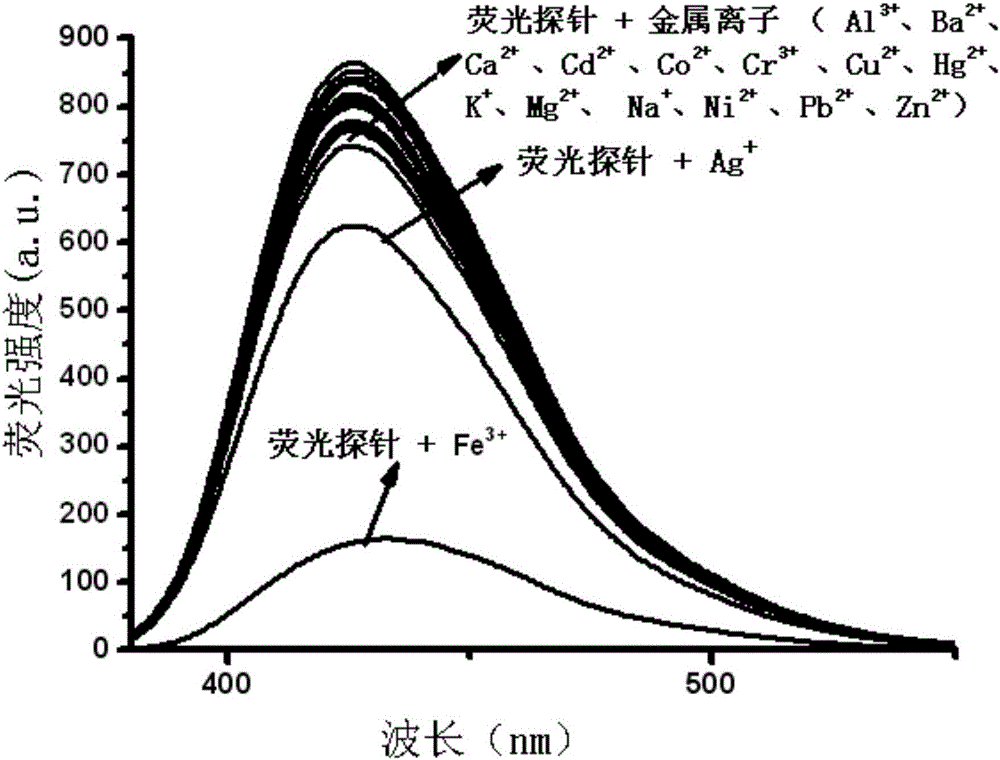
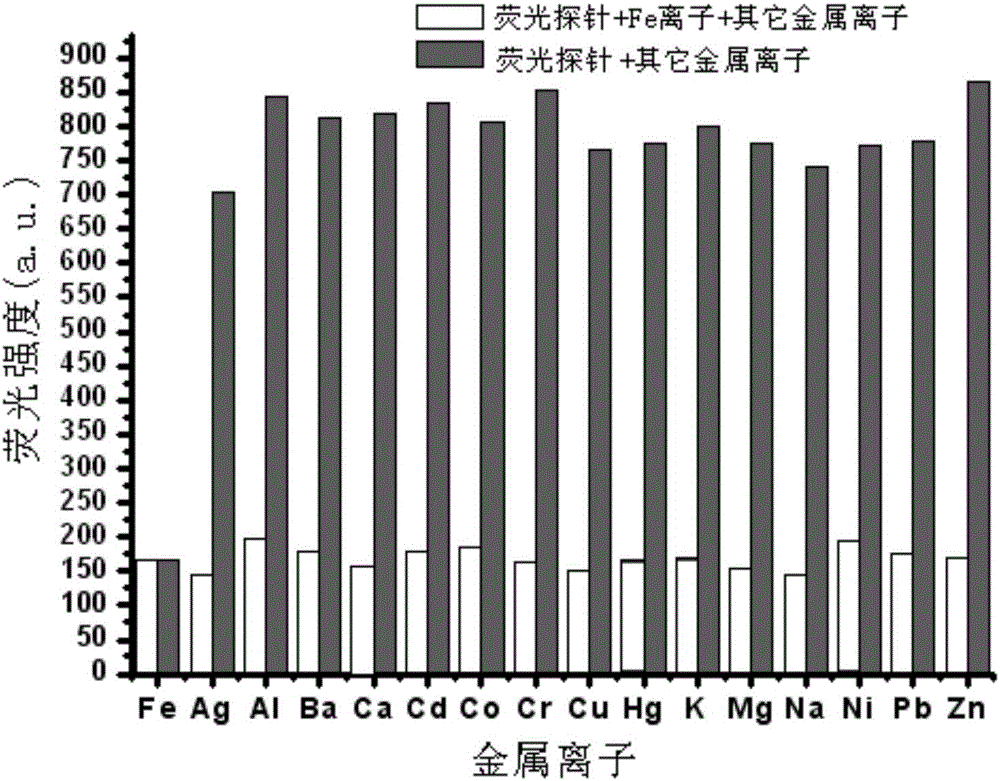
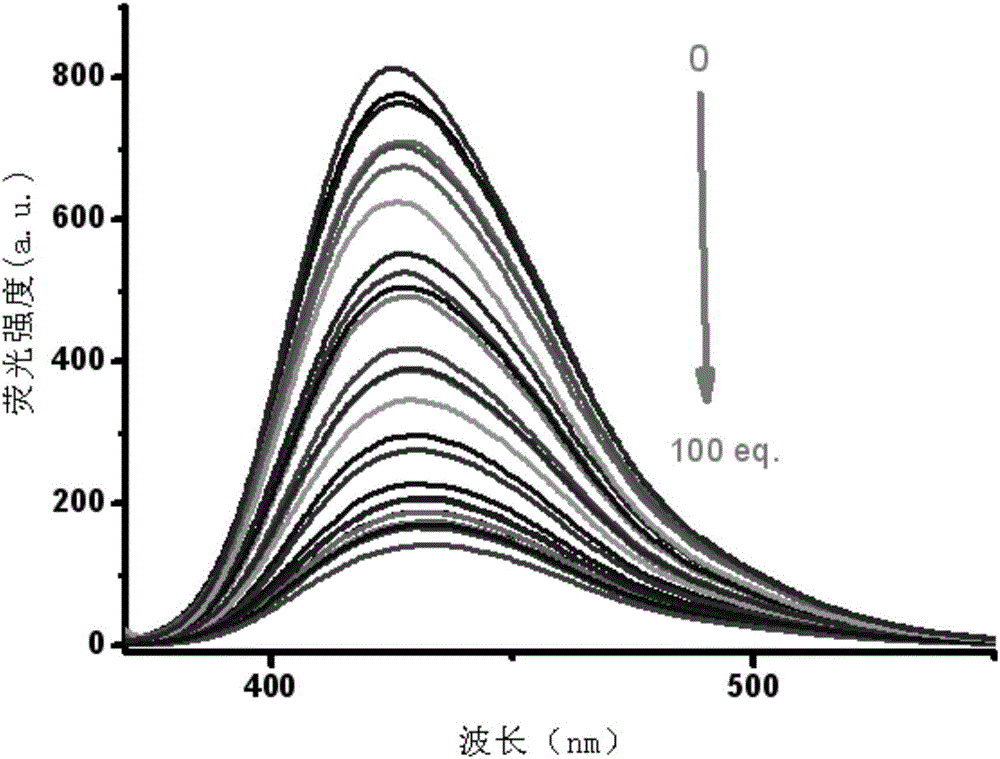
The invention relates to an Fe<3+> fluorescent probe and a preparation method thereof, particularly a bis-Schiff-base-connected symmetrical phenanthroimidazole Fe<3+> fluorescent probe and a preparation method thereof. The invention aims to solve the technical problems that the existing Fe<3+> fluorescent probe needs an organic solvent identification environment and copper ions can interfere with the identification of iron ions. The structural formula of the bis-Schiff-base-connected symmetrical phenanthroimidazole Fe<3+> fluorescent probe is disclosed in the specification. The preparation method comprises the following steps: 1. synthesizing an intermediate compound I from phenanthrenequinone, o-nitrobenzaldehyde, aniline and ammonium acetate; 2. synthesizing an intermediate compound II from the intermediate compound I, Raney nickel and hydrazine hydrate; and 3. synthesizing the Fe<3+> fluorescent probe from the intermediate compound II and terephthalaldehyde under acidic conditions. The Fe<3+> fluorescent probe can selectively identify Fe<3+> in a water-phase system within the wide pH value range of 1-9; the response time is 2 minutes; and when being used for Fe<3+> inspection, the Fe<3+> fluorescent probe has the advantage of no interference, and is convenient and quick.
Owner:QIQIHAR UNIVERSITY
Simple and efficient ambroxol synthesis method
ActiveCN102351720AReduce pollutionIncrease profitOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsEvaporation
The invention discloses a simple and efficient ambroxol synthesis method. In the method, a target product is obtained by three unit reactions, namely reduction, bromination and reductive amination. The method comprises the following specific ways: reacting based on o-nitrobenzaldehyde as a raw material and ethanol as a solvent in the presence of iron powder and trace concentrated hydrochloric acid so as to generate o-aminobenzaldehyde; brominating o-aminobenzaldehyde in the presence of bromine and hydrogen peroxide to obtain 3,5-dibromo-2-aminobenzaldehyde; reacting 3,5-dibromo-2-aminobenzaldehyde with trans-p-aminocyclohexanol in methanol for a while and then adding sodium borohydride for reaction so as to produce ambroxol; and finally, extracting out the target product through rotary evaporation. According to the invention, the bromination process is carried out under the mild reaction condition and has short reaction time and mild reaction condition; and 30% hydrogen peroxide and bromine are utilized to replace the traditional bromine salt, thereby improving the utilization rate of bromine, reducing the generation of organic byproducts and reducing pollution to environment.
Owner:NANJING UNIV OF SCI & TECH
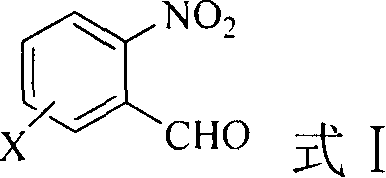
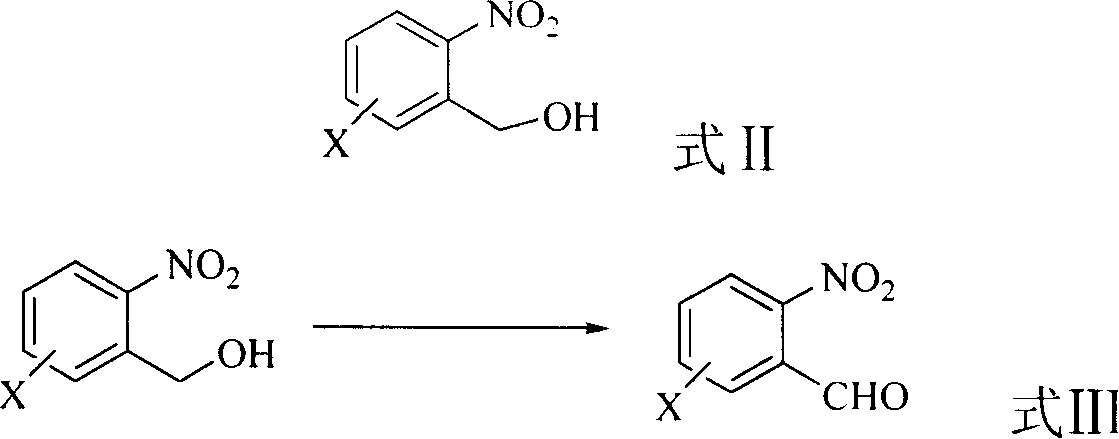
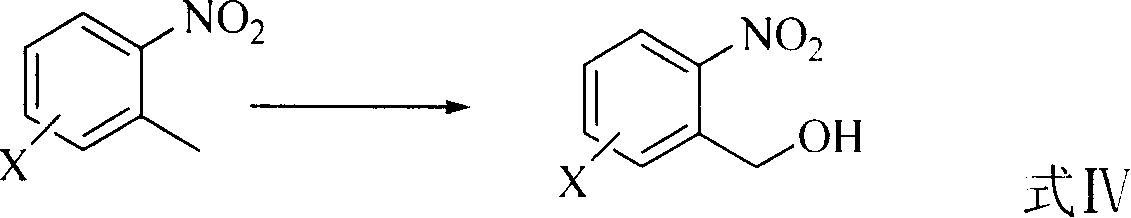
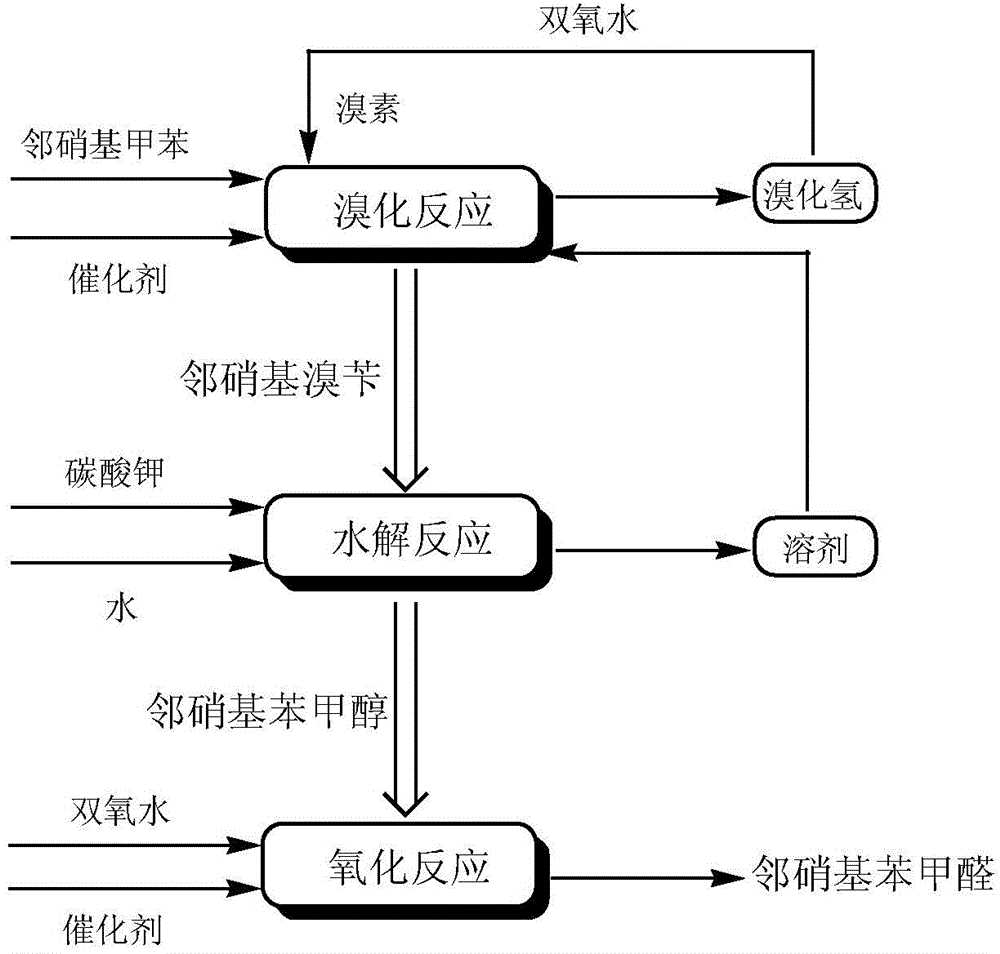
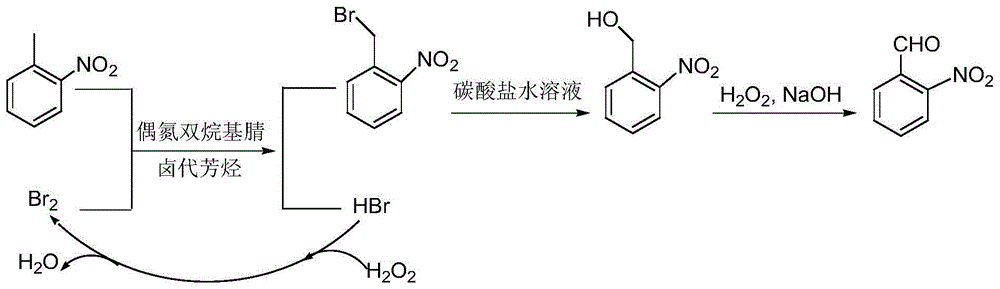
A preparing method of 2-nitrobenzaldehyde
InactiveCN105439867AEasy to cleanReduce pollutionOrganic chemistryOrganic compound preparationCatalytic functionOperation safety
The invention discloses a preparing method of 2-nitrobenzaldehyde. 2-nitrotoluene is adopted as a raw material and is brominated with bromine under catalytic function of azo-bis alkyl nitrile to generate 2-nitrobenzyl bromide and hydrogen bromide. The 2-nitrobenzyl bromide is hydrolyzed under catalytic function of an aqueous carbonate solution to generate 2-nitrobenzyl alcohol. The 2-nitrobenzyl alcohol is oxidized with hydrogen peroxide under catalytic function of sodium hydroxide to generate the objective compound, namely the 2-nitrobenzaldehyde. A hydrogen peroxide oxidation manner is adopted by the method, thus improving cleanliness of industrial preparation reactions, and reducing environment pollution. Oxidation is catalyzed by adopting the inorganic solid alkali catalyst and no metal organic complex catalyst is used, thus improving reaction stability and greatly reducing the cost of industrial preparation. An azo-bis alkyl nitrile solid catalyst in place of a peroxydicarbonate liquid catalyst is adopted to catalyze the bromination, thus improving reaction operation safety of industrial preparation. The method increases the product yield. The yield of the method is increased by about 5% than that of traditional industrial methods at present. The total yield can reach 77% and product purity is higher than 99%.
Owner:NANJING UNIV OF SCI & TECH
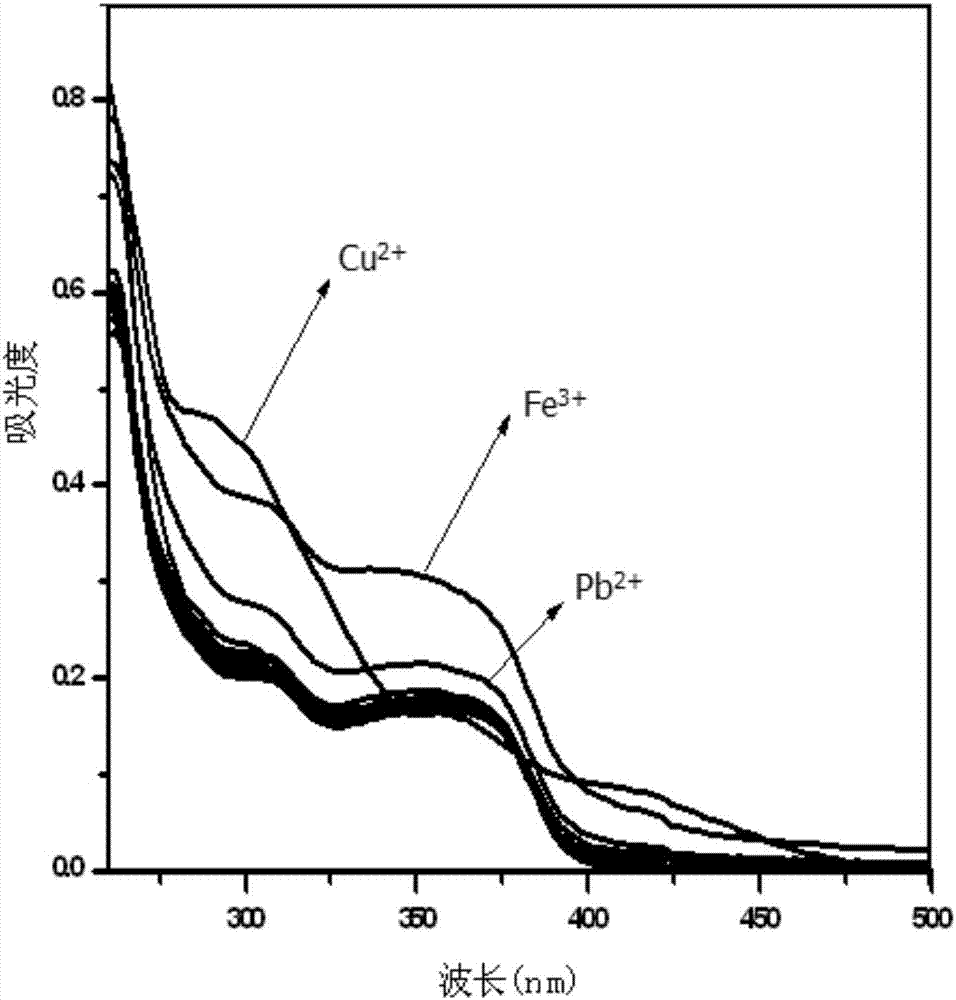
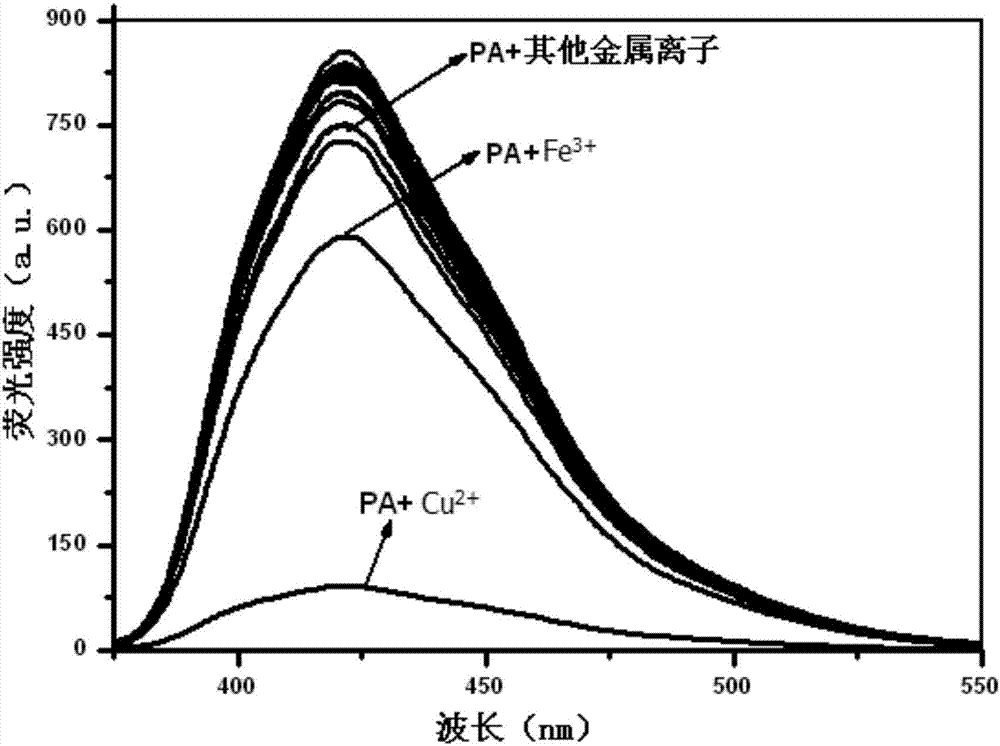
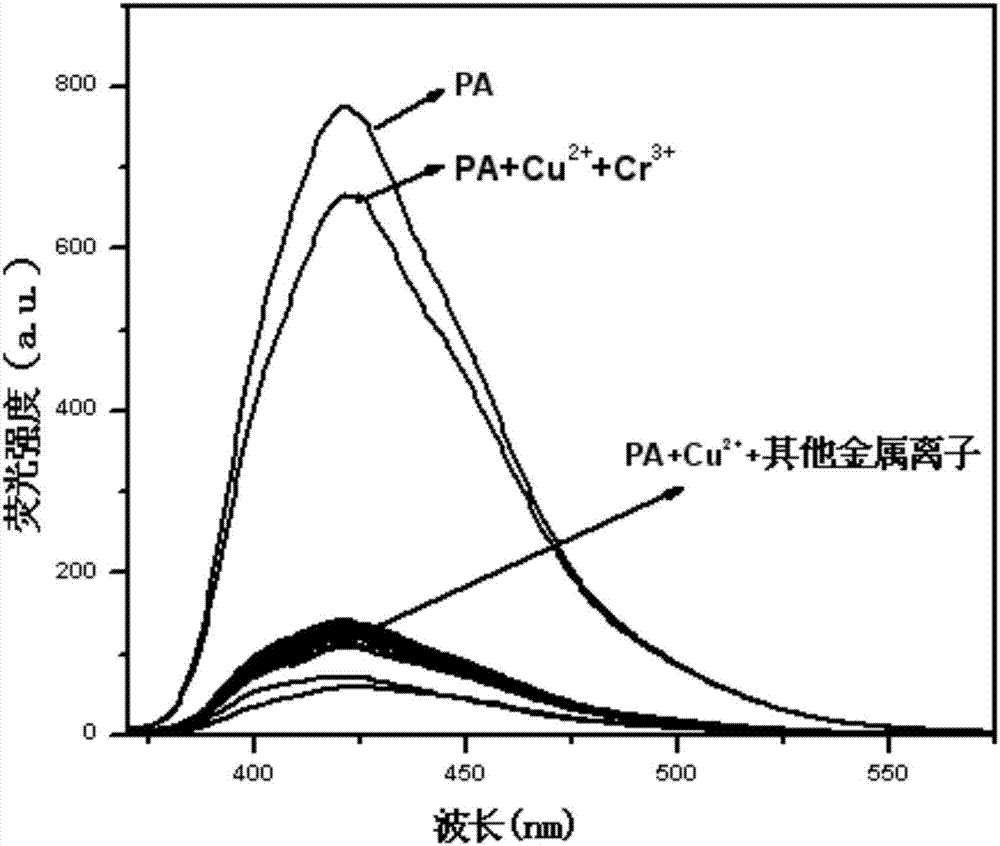
Phenanthrene imidazole reversible fluorescence probe for Cu<2+> detection, and preparation method and application thereof
InactiveCN106883183AHigh selectivityIncreased sensitivityOrganic chemistryFluorescence/phosphorescenceSalicylaldehydeStructural formula
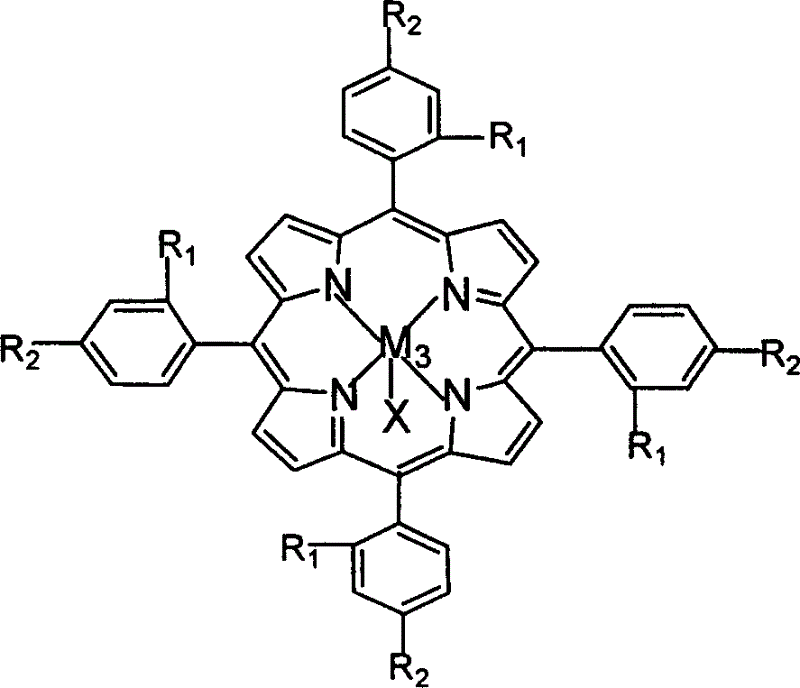
The invention relates to a phenanthrene imidazole reversible fluorescence probe for Cu<2+> detection, and a preparation method and application thereof, relates to the fluorescence probe for the Cu<2+> detection, and the preparation method and the application thereof, and aims to solve the technical problems of an existing fluorescence molecular probe is poor in Cu<2+> detection reversibility and easy to be interfered by other metal ions. The reversible fluorescence probe provided by the invention has a structural formula as shown in the figure. The preparation method comprises the steps of (1) using phenanthrenequinone, o-nitrobenzaldehyde and ammonium acetate for synthesizing an intermediate compound I; (2) using the intermediate compound I, raney nickel, ethanol and hydrazine hydrate for synthesizing an intermediate compound II; (3) reacting the the intermediate compound II and salicylaldehyde, and obtaining the phenanthrene imidazole reversible fluorescence probe for the Cu<2+> detection. During detection, after the reversible fluorescence probe is acted with Cu<2+>, the fluorescence intensity is quenched; after Cr<3+> is added, the fluorescence intensity is recovered and can be used for detecting pollution of the Cu<2+> and the Cr<3+> in water.
Owner:QIQIHAR UNIVERSITY
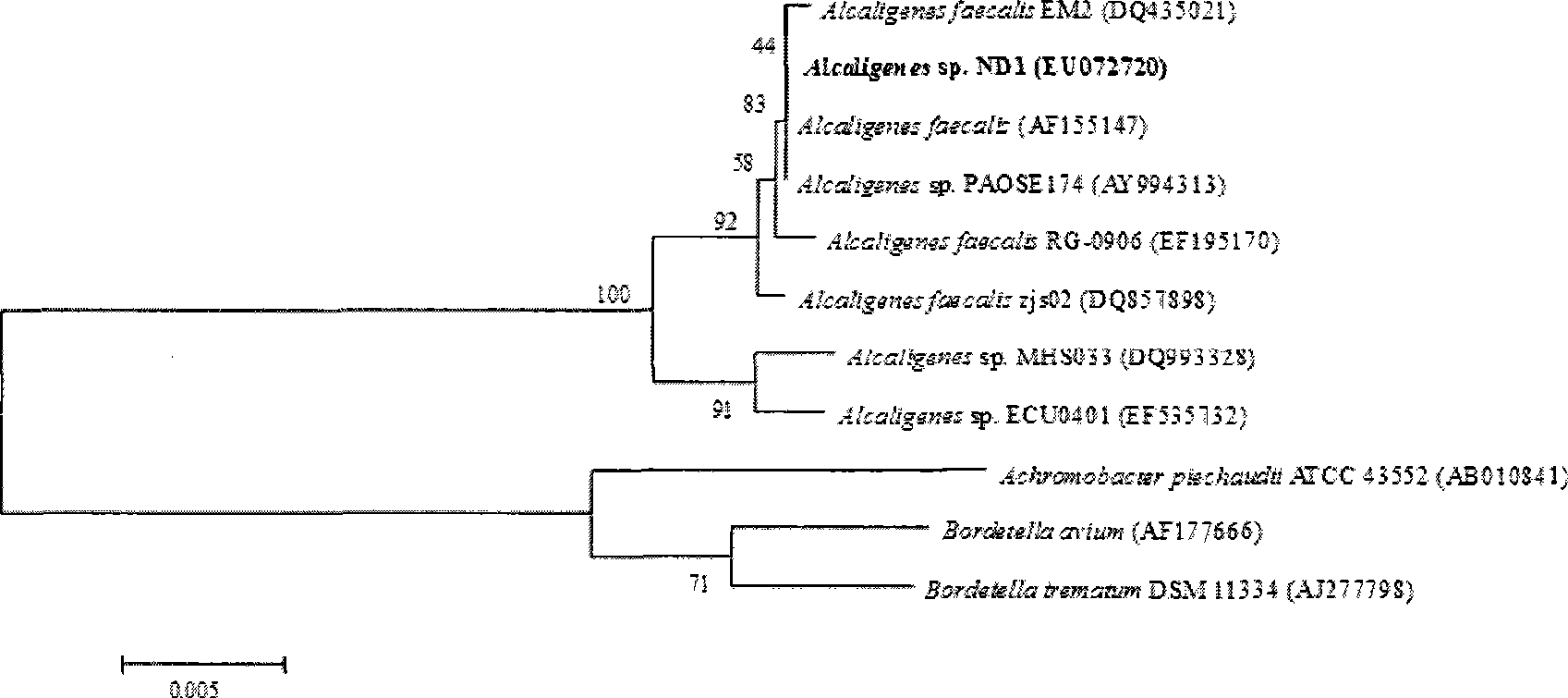
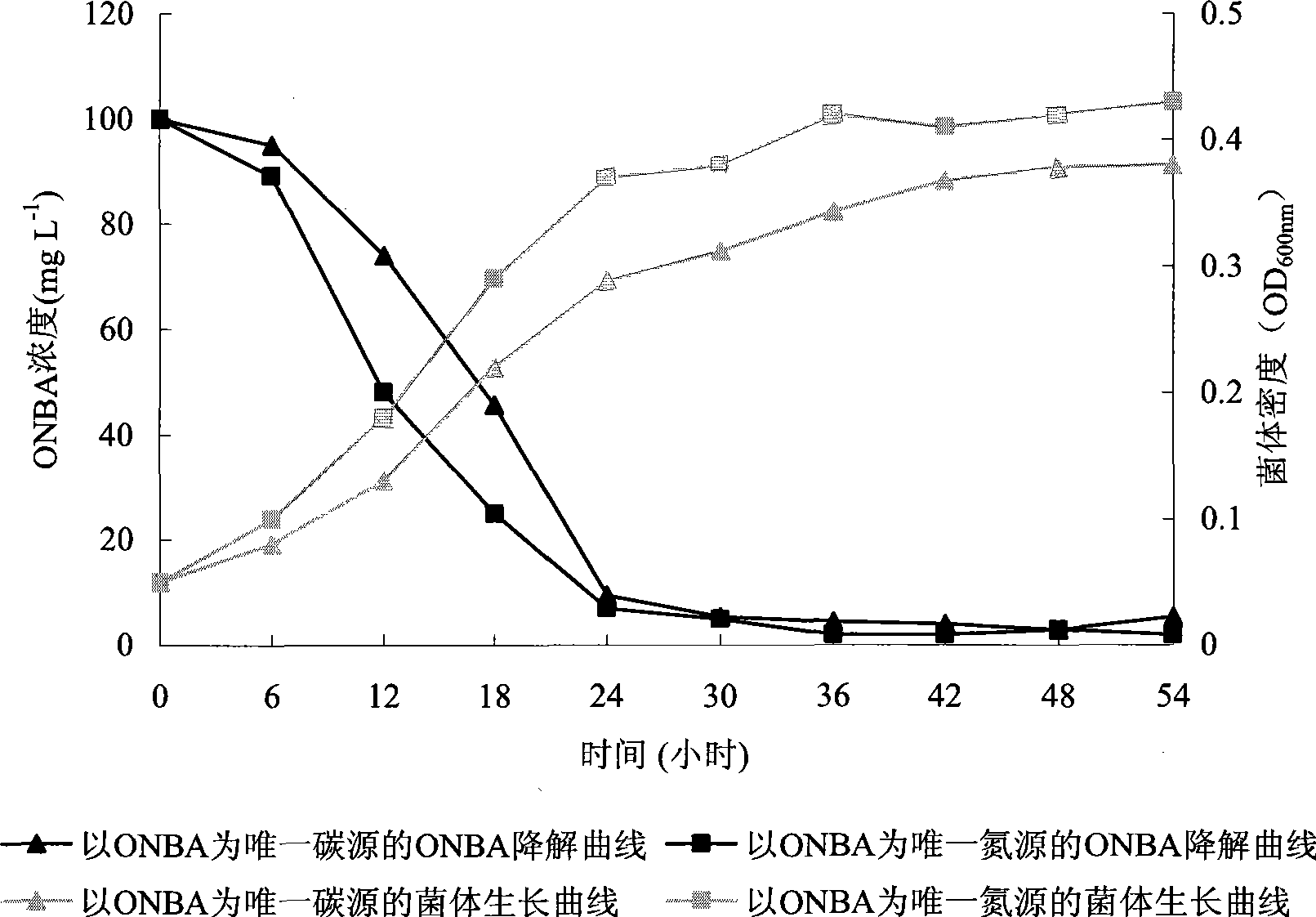
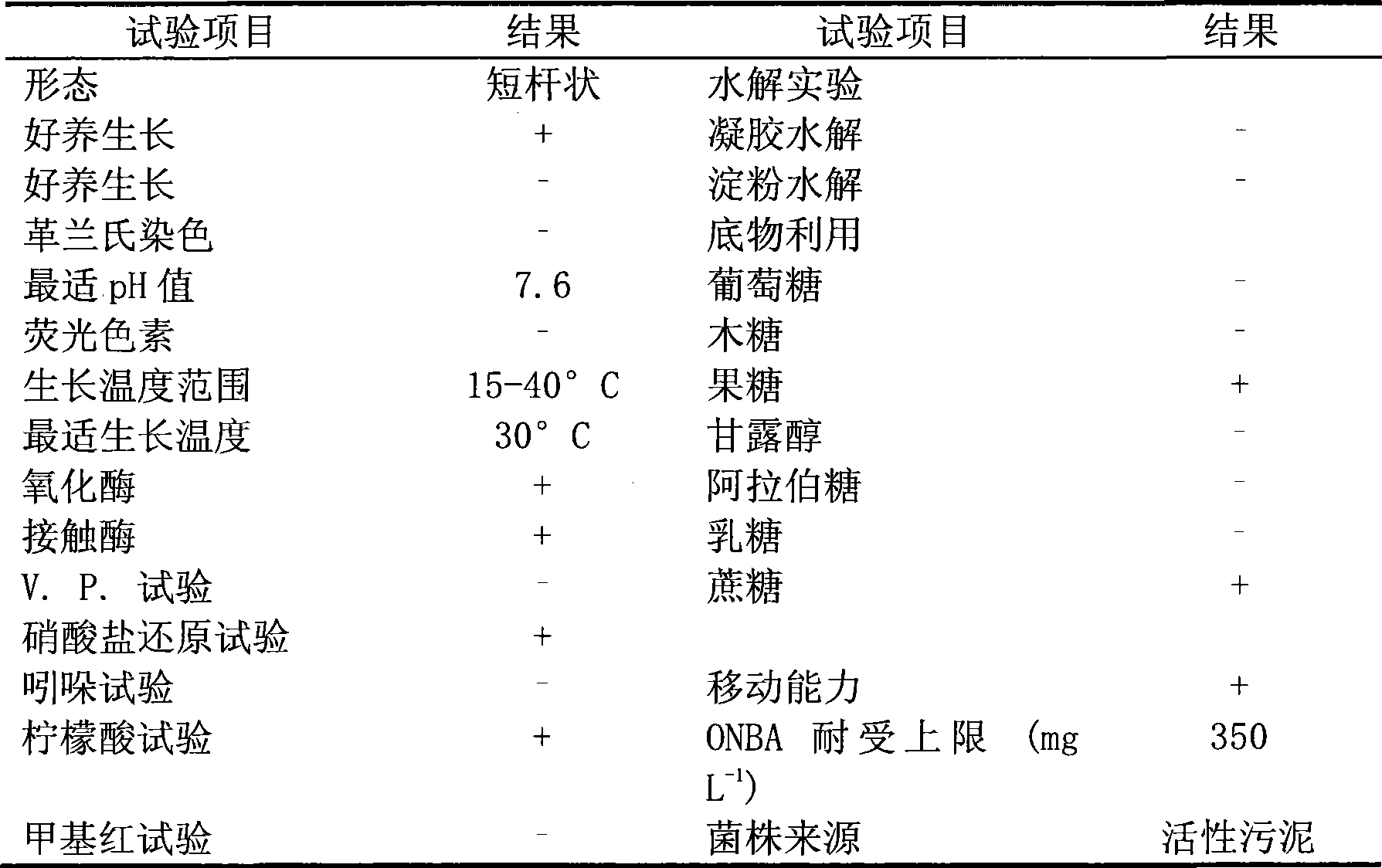
O-nitrobenzaldehyde degrading bacteria and use thereof
The invention pertains to the technical field of bio-treatment of environmental pollutants, particularly relates to a bacterium for degrading o-nitrobenzaldehyde and the applications thereof in bio-treatment of waste water and remediation of soil environmental pollution. The degrading bacterium of o-nitrobenzaldehyde is the strain ND1 of Alcaligenes sp. The bacterium can degrade o-nitrobenzaldehyde with high efficiency under aerobic condition, and utilize albocarbon, p-nitrophenol, o-nitrophenol, ortho-aminophenol, toluene, p-dimethylaminobenzaldehyde, 2, 4-dinitrophenol, para hydroxy benzoic acid, diphenylamine, benzoic acid, xylene and a plurality of other aromatic compounds. The Alcaligenes sp.ND1 is sensitive to five common antibiotics of tetracycline, kanamycin, streptomycin, chloromycetin and ampicillin, thus providing safe guarantees in the application thereof in the bio-treatment of waste water and remediation of soil environmental pollution.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Prepn process of nifedipine
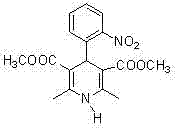
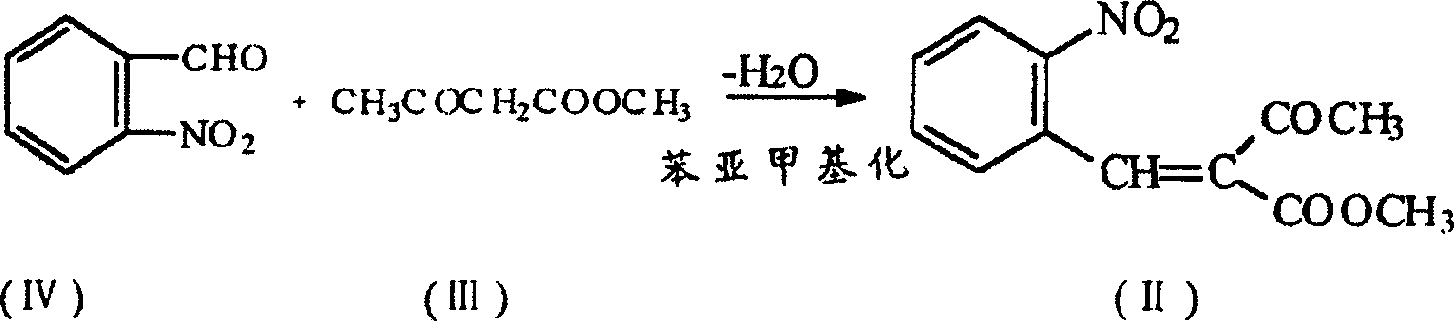
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde,the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
Synthesizing method for o-nitrobenzaldehyde compound
ActiveCN103467300AHigh yieldLow priceOrganic chemistryOrganic compound preparationOrganic acidBenzene
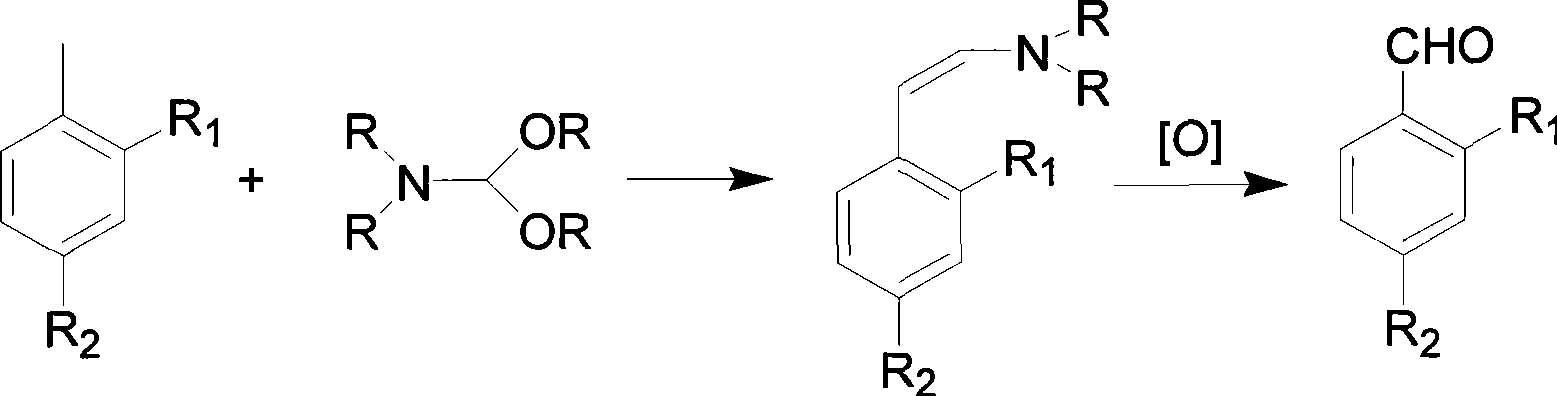
The invention provides a preparation method for an o-nitrobenzaldehyde compound. The method directly taking benzaldehyde compounds as starting raw materials comprises the following steps: firstly, converting a formyl group into an O-methyl oximido; secondly, taking divalent palladium salt as a catalyst, and realizing the carbon-hydrogen bond activation single nitration reaction on an o-position of an oximido under the condition that both an oxidant and a nitration agent exist; finally, removing the O-methyl oximido by using strong organic acid to obtain the o-nitrobenzaldehyde compound. The nitration method provided by the invention has the advantage of specificity in the o-position of a nitration position, the reaction process is safe and environment-friendly, the substrate is excellent in adaptability, and various substituents can realize o-position nitration; various benzaldehyde is directly taken as raw materials, so that the reaction steps are simple, and the synthesizing method is a novel route for synthesizing various o-nitrobenzaldehyde compounds containing substituents.
Owner:徐州宏阳新材料科技股份有限公司
O-nitrobenzaldehyde and p-nitrobenzaldehyde and preparation method of halides thereof
InactiveCN101362697AEasy to operateMild responseOrganic chemistryOrganic compound preparationOrtho positionPara-nitrobenzaldehyde
The invention discloses a preparation method for o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof, which comprises the following steps in sequence: toluene is used as raw material, wherein, ortho-position and para-position of toluene contain nitryl or nitryl and halogen. N,N-dialkyl formamide is used as solvent, and the raw material and the solvent are condensed with N,N-dialkyl formamide tirethylene ethanol to prepare N,N-dialkyl styrylamine, wherein, ortho-position and para-position of N,N-dialkyl styrylamine contain nitryl or nitryl and halogen; then, with the existence of phase transfer catalyst, the N,N-dialkyl styrylamine prepared is oxidized by taking peroxoic acid or hydrogen peroxide as oxidizer to produce o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof. By adopting the preparation method of the invention to prepare o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof, the operation is simple, the reaction conditions are easy to control, the raw material is easily available, the yield is higher, the product quality is stable and the purity is good.
Owner:ZHEJIANG UNIV
Prepn. process of nifedipine
The present invention is preparation process of nifedipine and relates to the field of organic chemical technology. Under the action of pyridine carboxylate in the catalytic amount, o-nitrobenzaldehyde and methyl acetoacetate are made to react to produce intermediate benzylidene compound, which is reacted with methyl acetoacetate and ammonia directly to produce nifedipine. In o-nitrobenzaldehyde, the total yield of re-crystallized nifedipine may reach 70%.
Owner:天津中安药业有限公司
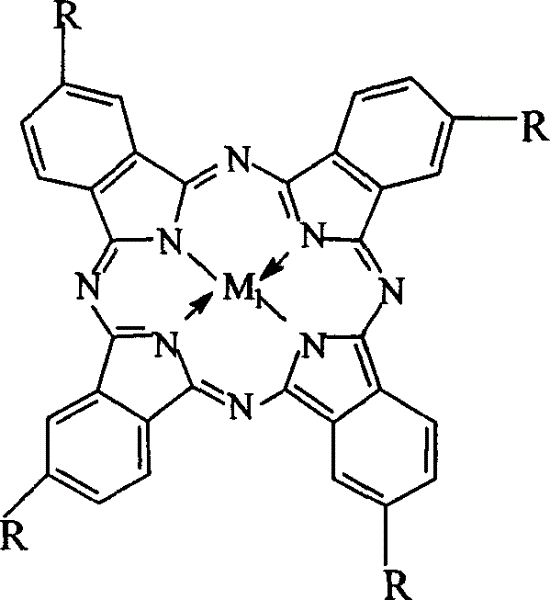
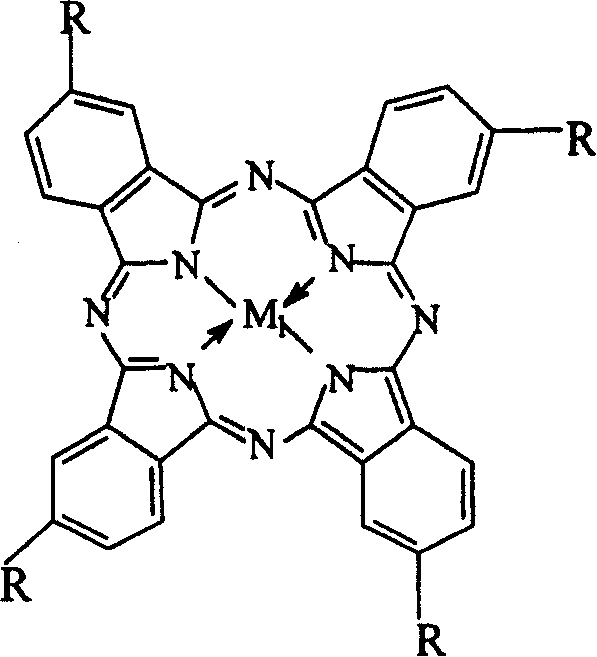
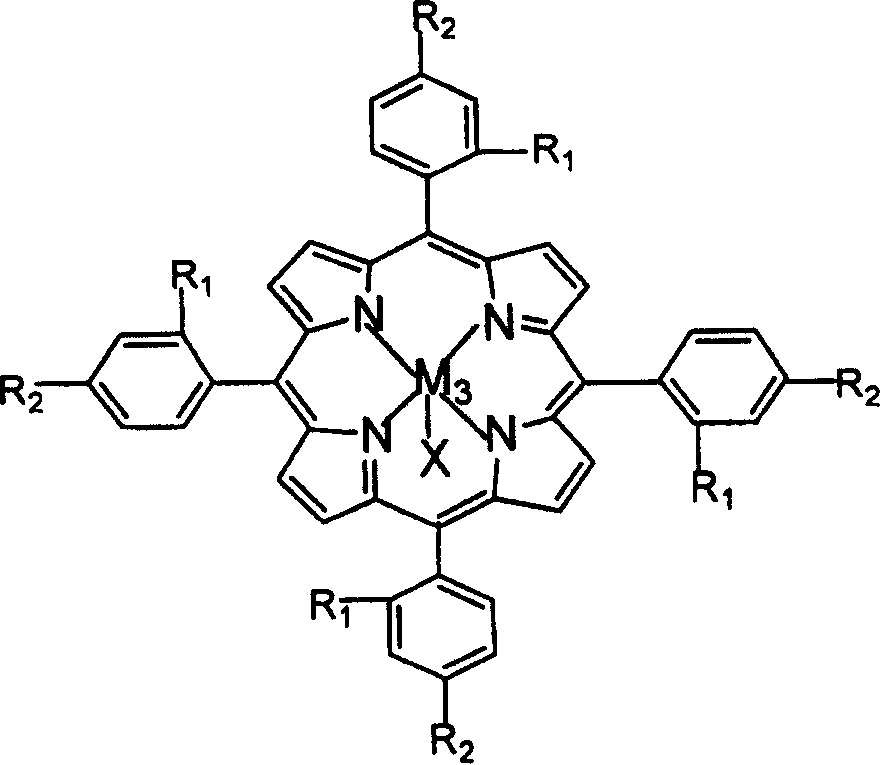
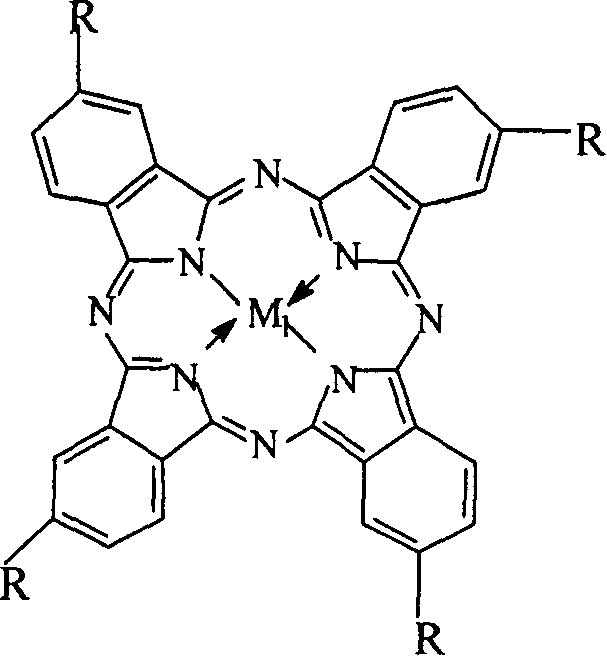
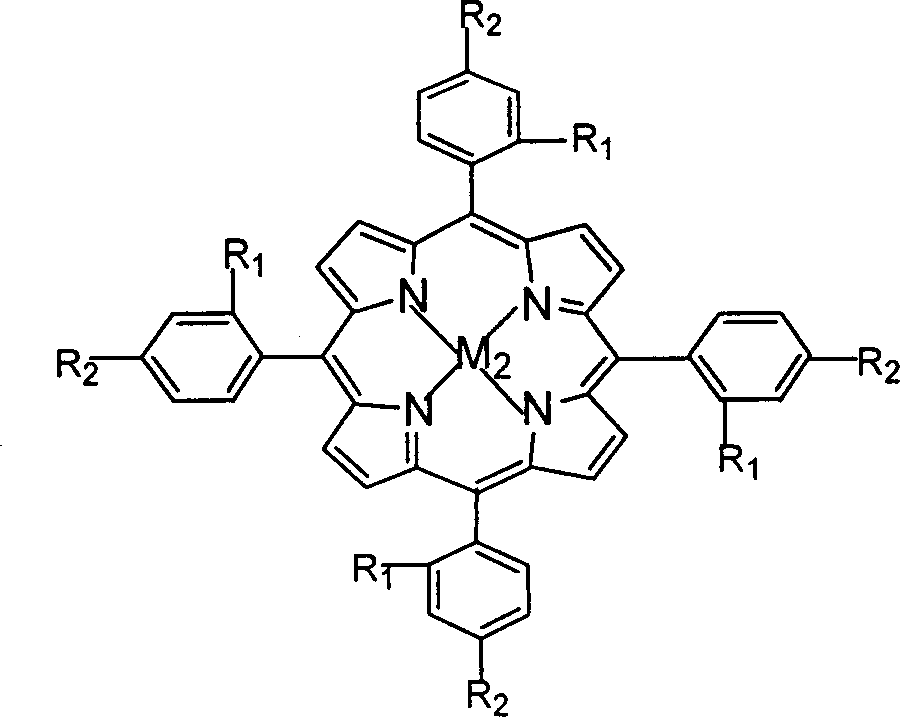
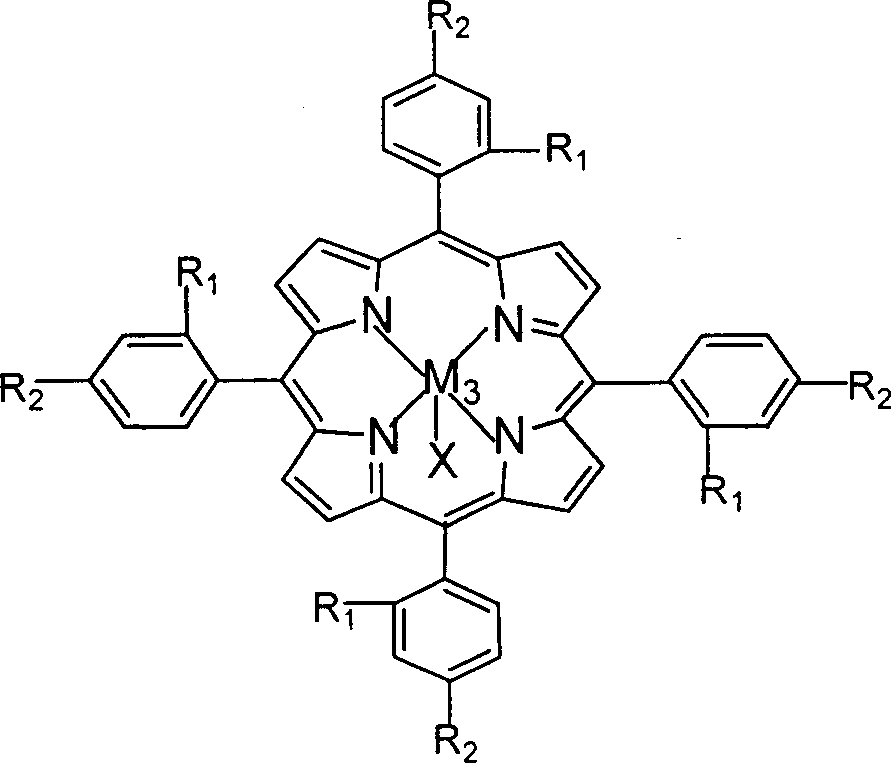
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546457AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
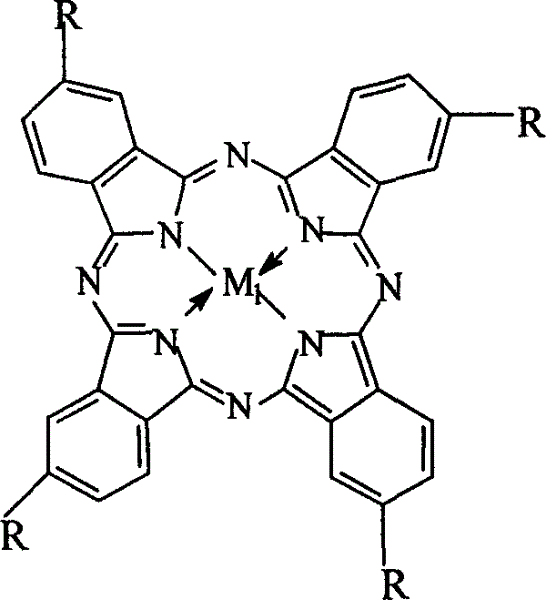
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing o-nitrobenzaldehyde through bionic catalytic oxidation of ortho-methylnitro benzene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.2-1.0% weight of ortho-methylnitro benzene, and methyl alcohol is used as solvent, 0.8-3.0 MPa oxygen is let into 3.0-6.0 mol / L strong alkaline methyl alcohol solution, the reaction temperature is controlled between 25 deg. C to 60 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
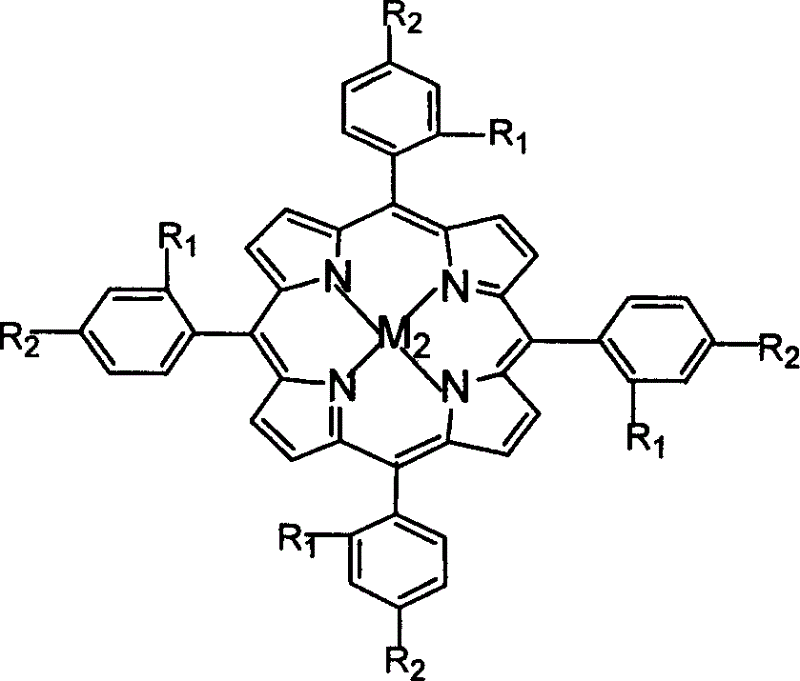
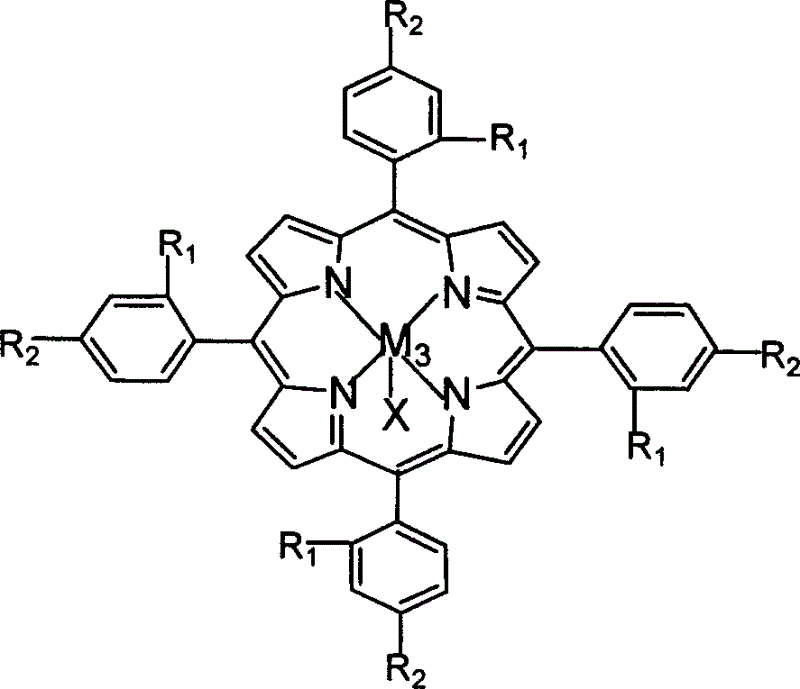
Light-response room-temperature phosphorescent supramolecular anti-counterfeit material and preparation method thereof
InactiveCN105802609AAchieving photoresponsivenessAdjust intensityGel preparationLuminescent compositionsLuminous intensityOrganic solvent
The invention provides a light-response room-temperature phosphorescent supramolecular anti-counterfeit material with adjustable luminous intensity and a preparation method thereof.The material is prepared in the mode that a gelator 1,3:24-benzylidene-sorbitol (DBS) serves as a self-assembly unit, and tetracarbonyl phenyl palladium porphyrins (Pd-TCPP) and o-nitrobenzaldehyde (NBA) are added into DBS and self-assembled into supramolecular gel in a mixed solvent composed of an organic solvent and water.The material has the advantages that before the supramolecular is irradiated by ultraviolet light, room-temperature phosphorescence cannot be generated by optical excitation; when the supramolecular is irradiated by ultraviolet light with the wavelength being 365 nm and the wavelength is excited to be 417 nm, room-temperature phosphorescence with the emission wavelength being 700 nm can be generated; the phosphorescence intensity of the supramolecular material can be controlled through the NBA concentration and ultraviolet light illumination time.The obtained supramolecular gel material has potential application value in the anti-counterfeit field.
Owner:HUAZHONG UNIV OF SCI & TECH
Nitrofurantoin residue enzyme-linked immunoassay kit
The invention discloses a nitrofurantoin residue enzyme-linked immunoassay kit which comprises a) an ELIAS plate, b) a standard solution, c) an antibody working solution, d) a cleaning solution, e) an enzyme marker, f) a substrate developing solution and g) a stopping solution, wherein the ELIAS plate is coated with nitrofurantoin metabolite AHD or coupling antigen of a derivative of the nitrofurantoin metabolite and a protein; and the standard solution is prepared by o-nitrobenzaldehyde which is dissolved in the dimethyl sulfoxide and then reacts with hydantoin derivative. The invention is mainly provided in a form of working solution, is characterized by convenient use, high specificity, high sensitivity, high precision, high accuracy and the like, and is applied to the detection of nitrofurantoin residues in animal food such as aquatic products and the like.
Owner:FOOD INSPECTION CENT OF CIQ SHENZHEN
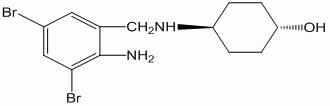
Synthesis method of ambroxol hydrochloride
ActiveCN104788326AReaction raw materials are readily availableEasy to produceOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsO-nitrobenzaldehyde
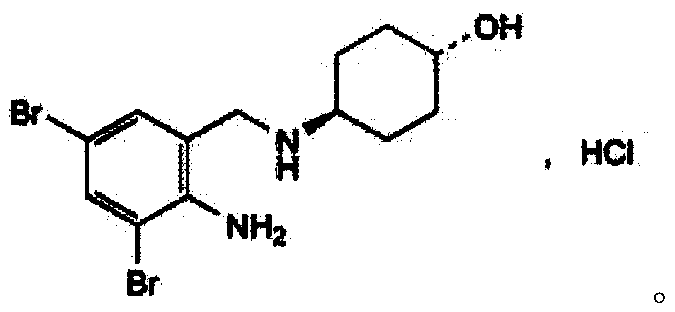
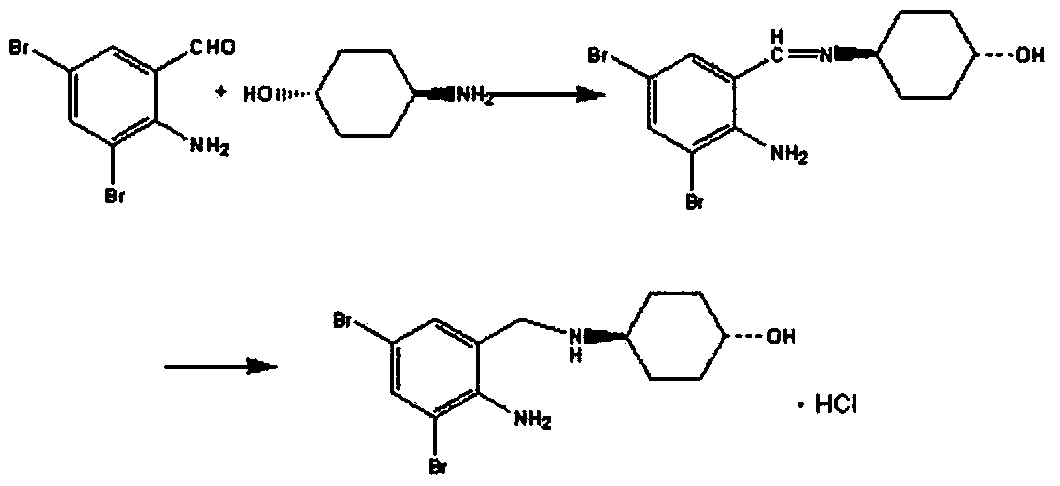
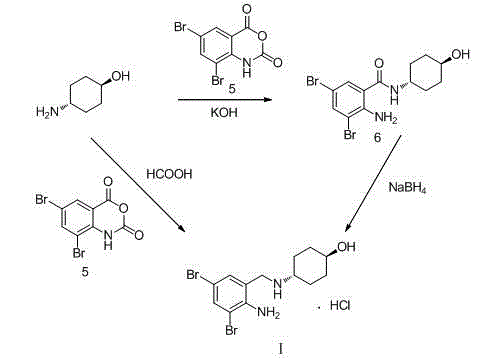
The invention belongs to the field of pharmaceutical synthesis and particularly discloses a synthesis method of ambroxol hydrochloride. The synthesis method comprises the steps of brominating o-nitrobenzaldehyde serving as a starting raw material to obtain 2-nitro-3, 5-dibromobenzaldehyde; reacting the obtained 2-nitro-3, 5-dibromobenzaldehyde and trans-4-aminocyclohexanol; and then, reducing and salifying hydrochloride to finally prepare ambroxol hydrochloride. The synthesis method disclosed by the invention is low in raw material cost, simple in operation, safe and environment-friendly, capable of simplifying the production process and increasing the yield and purity of products and suitable for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
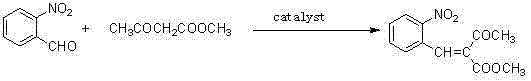
Preparation method of methyl 2-nitrobenzal acetoacetate
ActiveCN102976949AHigh purityMild reaction conditionsOrganic chemistryOrganic compound preparationNifedipineAcetic acid
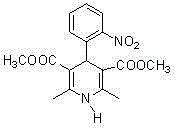
The invention provides a preparation method of methyl 2-nitrobenzal acetoacetate, which comprises the following steps: (1) reacting initial raw materials o-nitrobenzaldehyde and methyl acetoacetate with methanol under the action of a catalyst to generate a compound methyl 2-nitrobenzal acetoacetate; (2) after carrying out vacuum concentration to remove the back, adding a crystallizing solvent, and stirring at low temperature to generate the methyl 2-nitrobenzal acetoacetate solid crude product; and (3) recrystallizing the solid crude product to obtain the methyl 2-nitrobenzal acetoacetate pure product of which the purity is higher than 99.5%. The preparation method provided by the invention has the advantages of mild reaction conditions, short reaction time, high yield and environmental protection; the obtained nifedipine has high purity of the impurity methyl 2-nitrobenzal acetoacetate; and the impurity can be accurately positioned by comparison in the nifedipine related substance inspection, thereby having important instruction meanings for researching nifedipine.
Owner:QINGDAO HUANGHAI PHARM CO LTD
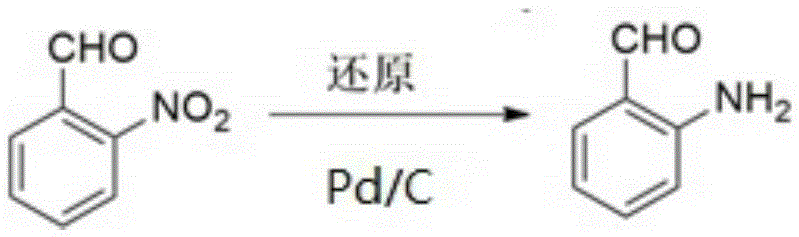
Method for synthesizing o-aminobenzaldehyde
InactiveCN105254516AImprove conversion rateHigh selectivityOrganic chemistryOrganic compound preparationReaction temperatureOrtho aminobenzaldehyde
The invention discloses a method for synthesizing o-aminobenzaldehyde. The method comprises the following steps: adding a Pd / C or Ni / C catalyst into a mixed solvent containing o-nitrobenzaldehyde; replacing air in a high-pressure reaction kettle with N2, and introducing H2 to react at 50-120 DEG C for 5-10 hours; filtering reaction liquid, and analyzing filtrate by virtue of a high performance liquid chromatography, wherein the conversion rate and the selectivity can reach 90% or more. According to the method, the catalyst can be recycled after the reaction; no waste liquid and waste residue is emitted in the reaction process; aldehyde is not reduced when nitryl is reduced.
Owner:LIANYUNGANG RES INST NANJING UNIV OF SCI & TECH
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1243717CEasy to operateSimple stepsOrganic chemistryOrganic compound preparationCatalytic oxidationPorphyrin
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing o-nitrobenzaldehyde through bionic catalytic oxidation of ortho-methylnitro benzene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.2-1.0% weight of ortho-methylnitro benzene, and methyl alcohol is used as solvent, 0.8-3.0 MPa oxygen is let into 3.0-6.0 mol / L strong alkaline methyl alcohol solution, the reaction temperature is controlled between 25 deg. C to 60 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Novel disinfectant fluid and preparation method thereof
InactiveCN102870808AAlleviate re-breedingRaw materials are simpleInorganic/elemental detergent compounding agentsBiocideHazardous substanceDisinfectant
The invention discloses a novel disinfectant fluid. The novel disinfectant fluid comprises the following raw materials in part by weight: 2 to 3 parts of disodium edetate, 10 to 18 parts of potassium iodide, 1 to 2 parts of sodium dodecylsulfate, 10 to 20 parts of azelaic acid, 3 to 5 parts of o-nitrobenzaldehyde, 5 to 8 parts of butyl glycol ether, and 10 to 18 parts of water. The novel disinfectant fluid provided by the invention has the advantages that the raw materials are simple and low cost; the novel disinfect fluid does not contain harmful substances such as phosphor and is environment-friendly; the novel disinfectant fluid has complete functions and can be used for cleaning, disinfection and sterilization; and the novel disinfectant fluid has a good using effect, can realize quick disinfection, and can be used to abate the rebreeding of bacteria.
Owner:QINGDAO SANDING SANITARY PROD
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1271040CNo pollution in the processLow costOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase
The invention belongs to the technical field of organic chemical, and in particular relates to a method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase. The structure of the compound is characterized and is confirmed by the methods of 1HNMR, 13CNMR, IR, MS and the like. The method is as follows: 1, 3-dipolar quinazoline dipole prepared by reaction of o-nitrobenzaldehyde, triethyl orthoformate and a series of compounds reacts with terminal alkyne under mild conditions in the water phase, and a series of pyrazolo [1, 5-c] quinazoline derivatives can be prepared by reaction at the temperature of 70 DEG C under the conditions of using copper sulfate pentahydrate as a catalyst and DBU (diazabicyclo) as an alkali. The method can effectively prepare the pyrazolo [1, 5-c] quinazoline skeleton compounds. The method has the advantages of mild reaction condition, simple operation, low cost, less side reaction, green and environmentally friendly reaction conditions, high purity of product, and easy separation and purification, and can be suitable for large scale preparation, and due to broad spectrum biological activity, the quinazoline skeleton compounds have the very good application prospect in the research and development of new drugs.
Owner:JIANGXI NORMAL UNIV
Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition
InactiveCN104610267AReaction conditions are green and environmentally friendlyLow costOrganic chemistryNitrostyrolNitrobenzene
The invention belongs to the technical field of organic chemistry, and particularly relates to a method for efficiently synthetizing pyrazol[1,5-c]-quinazoline skeleton compounds under no catalytic condition. The structure of the compounds is represented and confirmed with 1H NMR, 13 C NMR, MS and other methods. The method disclosed by the invention comprises the following steps: reacting 1,3-dipolar quinazoline dipoles obtained from o-nitrobenzaldehyde and a series of compounds of triethyl orthoformate with beta-nitrostyrolene under the condition of no any catalysts based on DMSO as a solvent at the temperature of 110 DEG C to generate a series of pyrazol[1,5-c]-quinazoline derivatives. By adopting the method, the pyrazol[1,5-c]-quinazoline compounds can be efficiently prepared. The method disclosed by the invention is mild in reaction conditions and simple to oeprate, the cost is greatly lowered with comparison to the cost of previous reaction between the 1,3-dipolar quinazoline dipoles and terminal alkyne, the reaction conditions are environmentally friendly, the production purity is high, separation and purification are convenient, the method is suitable for large-scale preparation, and the pyrazol[1,5-c]-quinazoline skeleton compounds have excellent application prospect in new drug research and development since the structure of quinazoline compounds has broad-spectrum biological activity.
Owner:JIANGXI NORMAL UNIV
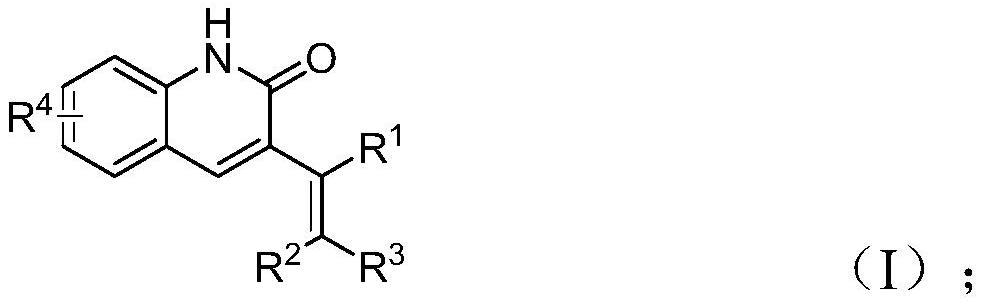
Preparation method of 3-alkenyl quinoline-2 (1H) ketone derivative
ActiveCN114478375AWide range of toleranceEasy to prepareOrganic chemistryAgainst vector-borne diseasesQuinolineEthylic acid
The invention discloses a preparation method of a 3-alkenyl quinoline-2 (1H) ketone derivative, which comprises the following steps: carrying out reaction on palladium acetate, tris (3-methoxyphenyl) phosphine, carbonyl molybdenum, cesium carbonate, tetrabutylammonium iodide, o-nitrobenzaldehyde and allyl aryl ether at 100 DEG C for 30 hours, and after the reaction is completed, carrying out post-treatment to obtain the 3-alkenyl quinoline-2 (1H) ketone derivative. According to the preparation method, o-nitrobenzaldehyde is used as a nitrogen source and a formyl source, the operation is simple, the reaction initial raw materials are cheap and easy to obtain, the tolerance range of substrate functional groups is wide, and the reaction efficiency is high. Various 3-alkenyl quinoline-2 (1H) ketone derivatives can be synthesized according to actual needs, and the practicability of the method is widened while the operation is convenient.
Owner:ZHEJIANG SCI-TECH UNIV
Computer keyboard disinfection cleaning solution
InactiveCN104140899AImprove the bactericidal effectAffect normal useInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsActive agentNitrobenzene
A computer keyboard disinfection cleaning solution comprises, by weight, 6-10 parts of lemon extracting solution, 3-8 parts of anion surface active agent, 3-6 parts of polytetrahydrofuran ether, 1-4 parts of glutaraldehyde, 0.5-2 parts of essence, 4-6 parts of sodium tripolyphosphate, 1-5 parts of sodium gluconate, 2-4 parts of citric acid, 3-5 parts of ethanediol, 0.6-2.2 parts of emulgator, 2.5-7 parts of o-nitrobenzaldehyde and 25 parts of water. The computer keyboard disinfection cleaning solution has the advantages that equipment can be well sterilized and disinfected through the disinfection cleaning solution, normal usage of the electronic device cannot be corroded or affected, and the disinfection cleaning solution is natural and friendly to the environment.
Owner:QINGDAO JIZHI ENERGY SAVING ENVIRONMENTAL PROTECTION
Method for synthesizing o-nitrobenzaldehyde compounds
ActiveCN101219955AEasy to operateMild reaction conditionsOrganic chemistryOrganic compound preparationAcetic acidSynthesis methods
Owner:SHANGHAI CHEMPARTNER CO LTD
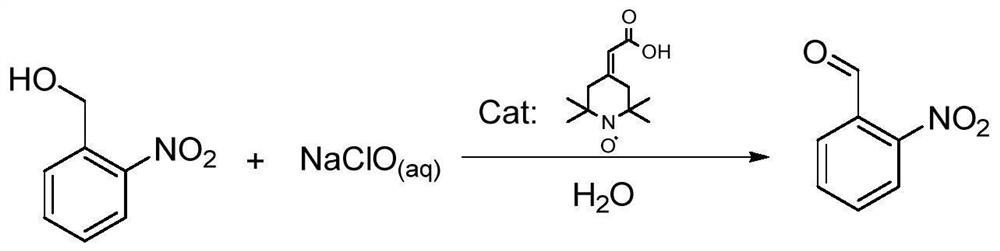
Preparation method of o-nitrobenzaldehyde
ActiveCN113087628AMild and moderate oxidationShort preparation timeOrganic chemistryOrganic compound preparationPtru catalystNitrobenzene
The invention discloses a preparation method of o-nitrobenzaldehyde, wherein the preparation method comprises the following steps: (1) adding o-nitrobenzyl alcohol, a catalyst, a bromide salt and a solvent into a reaction kettle, and uniformly stirring at room temperature; (2) controlling the reaction temperature, slowly dropwise adding a sodium hypochlorite solution into the reaction kettle, and simultaneously adding a weakly alkaline substance or an acidic substance to control the pH value of the reaction; (3) standing for layering, adding an organic solvent to extract a product, and concentrating to obtain a crude product; and (4) crystallizing by using an organic solvent, centrifuging, and drying in vacuum to obtain o-nitrobenzaldehyde. According to the method, 2-(2,2,6,6-tetramethylpiperidine nitroxide radical-4-subunit) acetic acid which is low in price and easy to obtain is used as a catalyst, the sodium hypochlorite solution is used as an oxidizing agent, a mixed solvent of water and chlorinated hydrocarbon is used as a reaction solvent, the o-nitrobenzaldehyde is prepared through oxidation under the mild temperature condition, the raw materials are low in price and easy to obtain, the reaction time is short, the reaction selectivity is high, and the yield is high.
Owner:陕西西岳制药(扶风)有限公司 +1
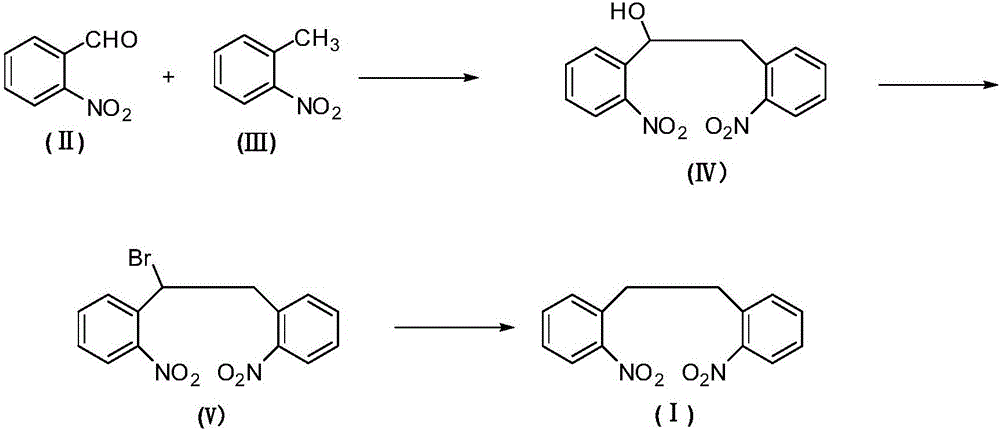
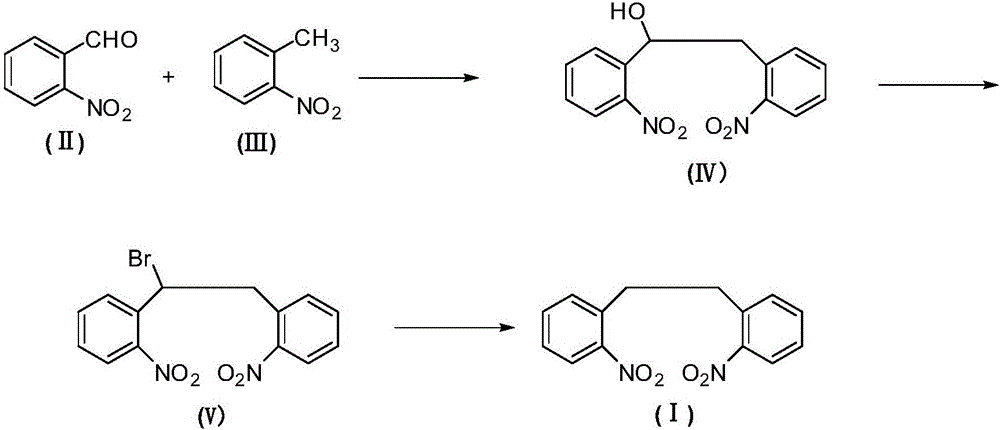
2,2'-dinitrodibenzyl preparation method
ActiveCN105753709AFacilitate the approach to sustainable developmentAchieve sustainable developmentOrganic chemistryOrganic compound preparationBromineSubstitution reaction
The invention relates to an antiepileptic drug carbamazepine intermediate preparation method, in particular to a 2,2'-dinitrodibenzyl preparation method.The 2,2'-dinitrodibenzyl preparation method includes the steps of subjecting o-nitrobenzaldehyde and o-nitrotoluene serving as raw materials to addition reaction then substitution reaction with a bromination reagent, and debromination under the action of a reducing agent so as to obtain 2,2'-dinitrodibenzyl.The 2,2'-dinitrodibenzyl preparation method has the advantages of a novel synthetic route, conventional reaction steps, simple technologies, high yield, high purity, capability of avoiding high-pollution operating steps and suitability for industrial production.
Owner:ANHUI JINDING PHARMA
Preparation method of methyl 2-nitrobenzal acetoacetate
ActiveCN102976949BHigh purityMild reaction conditionsOrganic chemistryOrganic compound preparationNifedipineAcetic acid
Owner:QINGDAO HUANGHAI PHARM CO LTD
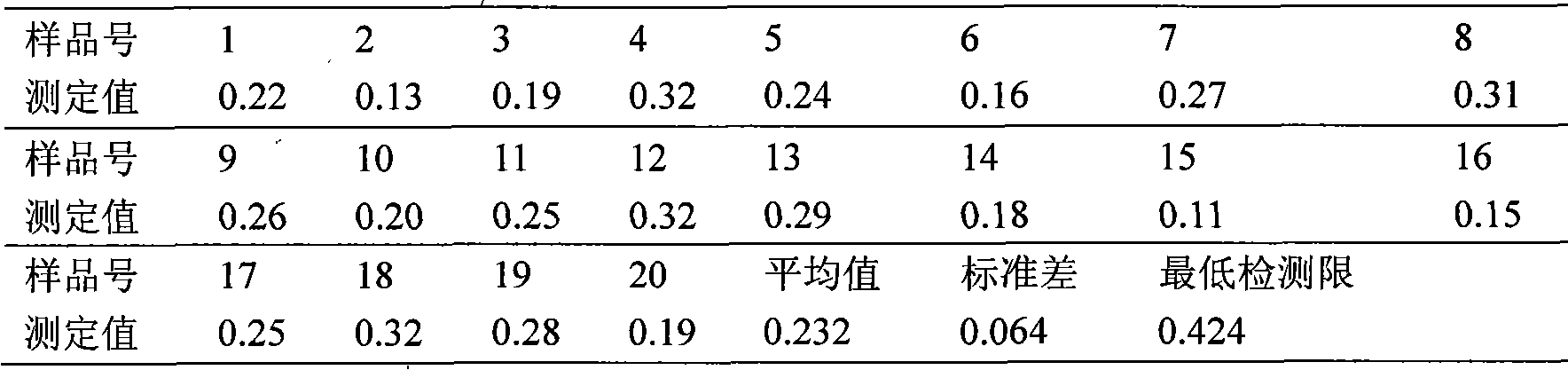
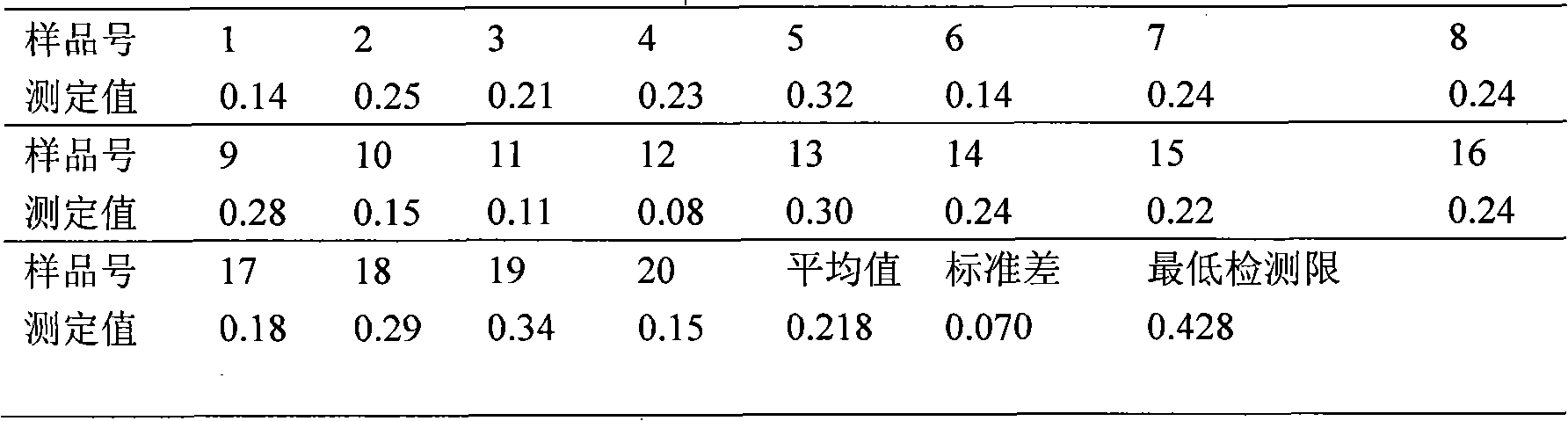
3,5-dinitrosalicylic acid hydrazine enzyme-linked immunologic detecting kit and its use method
ActiveCN101408544AHigh sensitivityStrong specificityBiological testingHydrazine compoundEthyl acetate
The invention discloses a 3, 5-dinitrosalicylic acid hydrazide enzyme-linked immunodetection test kit which comprises a box body, specific antibody solution, 3, 5-dinitrosalicylic acid hydrazine standard solution, an ELISA plate, sample diluent, washing fluid, second antibody solution, colored solution A, colored solution B and stopping solution; wherein, the specific antibody is a rabbit anti-3,5-dinitrosalicylic acid hydrazide antibody; and half antigens and ovalbumin conjugates are coated inside the hole of the ELISA plate; and the second antibody solution is horseradish peroxidase labeled goat anti-rabbit antibody diluent. After the sample to be tested is hydrolyzed by hydrochloric acid, derived by o-nitrobenzaldehyde, extracted by ethyl acetate, re-dissolved by the sample diluent and degreased by n-hexane, and the like, the test kit is used for detection and analysis. The test kit is strong in specificity, and the minimum detection limit is 74.8ppt, thereby being suitable for rapid detection of 3, 5-dinitrosalicylic acid hydrazine in feed, meat, urine, milk and other samples by the health, quality supervision and customs departments, meat processing plants and livestock farms.
Owner:SOUTH CHINA AGRI UNIV
Preparation method of 2-amino-3,5-dibromobenzaldehyde
InactiveCN105152947ASimple processReduce workloadOrganic chemistryOrganic compound preparationAcetic acidNitrobenzene
The invention discloses a preparation method of 2-amino-3,5-dibromobenzaldehyde. The yield of the method is higher (greater than 90 percent) and the purity is higher (greater than 99.0 percent). In the method, o-nitrobenzaldehyde is used as a raw material, nitro is reduced by using an iron powder / glacial acetic acid system to obtain o-aminobenzaldehyde mixture, and the mixture is not subjected to any treatment, is cooled and is directly dropped with bromine to obtain 2-amino-3,5-dibromobenzaldehyde. Since the intermediate product o-aminobenzaldehyde is not treated in the method, the process is simplified, the workload is reduced, the loss of o-aminobenzaldehyde during treatment is eliminated and the yield is improved; iron powder and bromine produce iron bromide, catalytic bromination is realized, the bromination efficiency is improved, the consumption of iron powder reduces the amount of iron sludge and the pollution is reduced.
Owner:JIANGSU QINGJIANG PHARMA
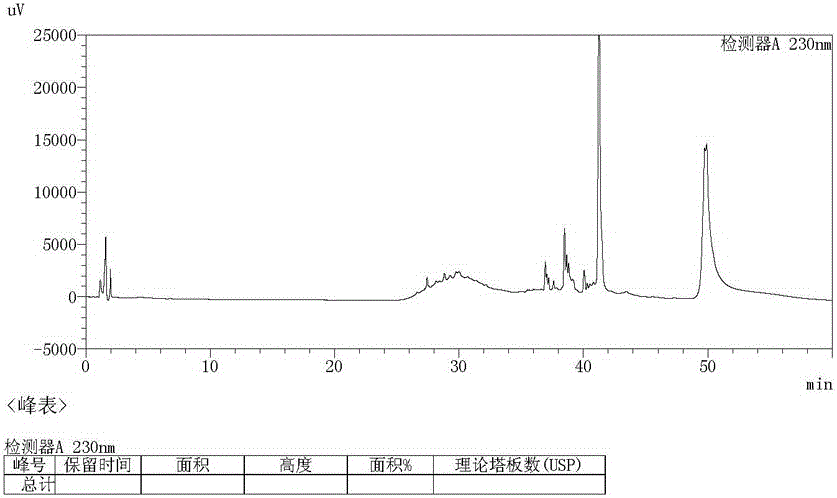
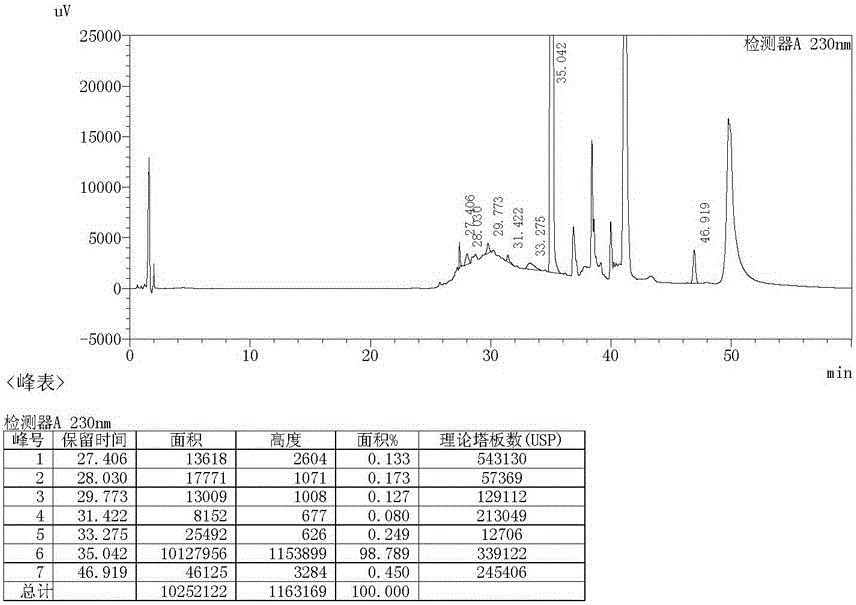
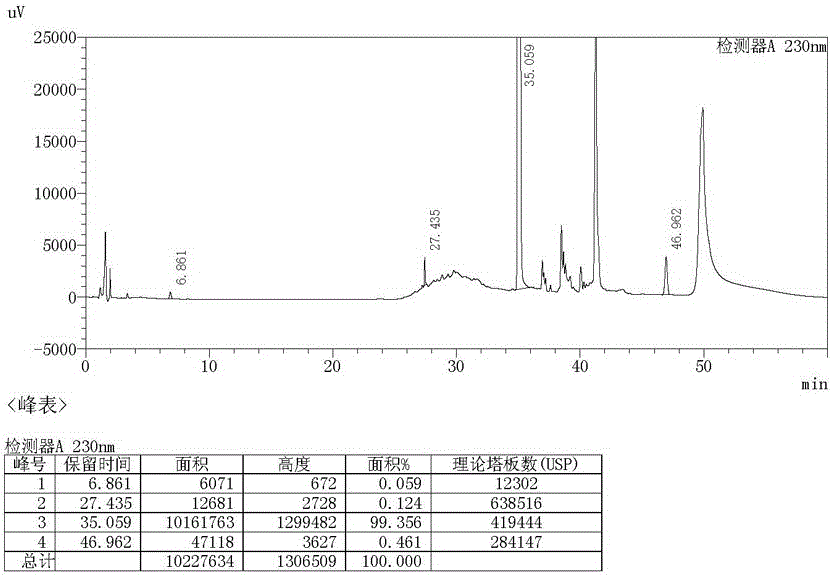
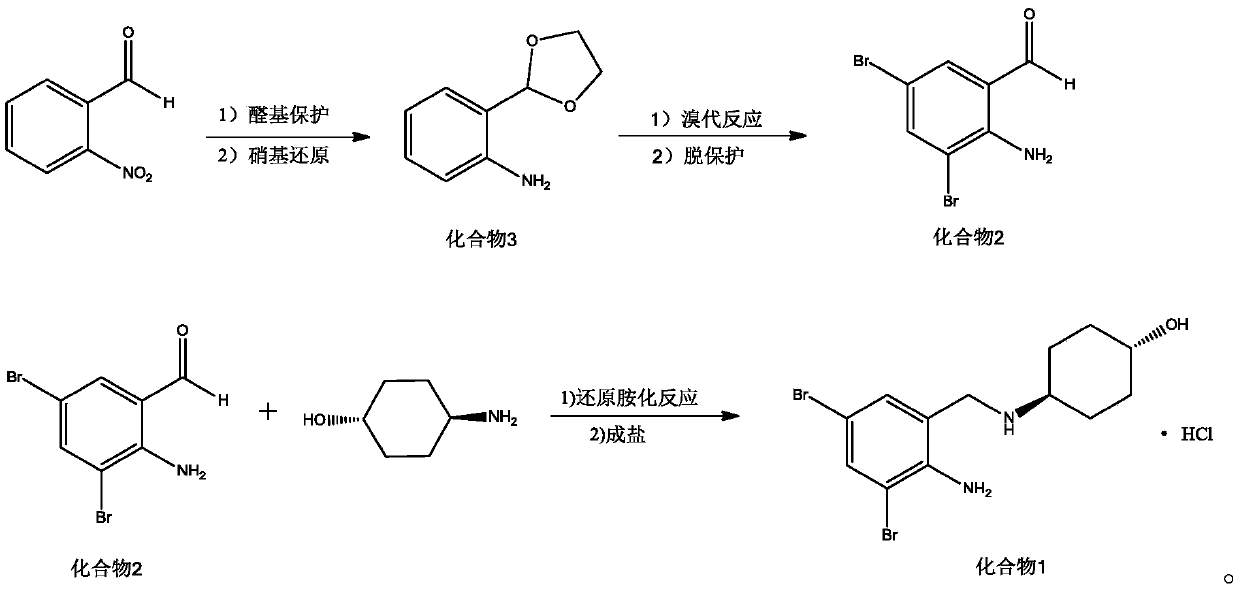
Preparation method of ambroxol hydrochloride
ActiveCN111072500AThe reaction steps are simpleHigh reaction yieldOrganic compound preparationOrganic chemistry methodsPtru catalystNitrobenzene
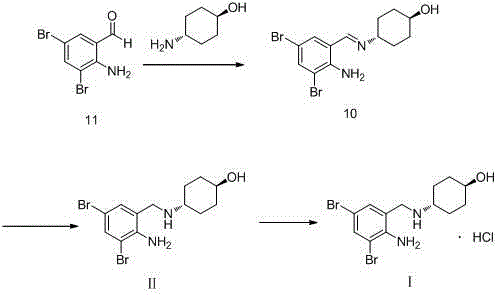
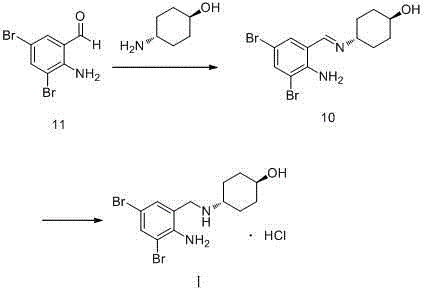
The invention relates to a preparation method of ambroxol hydrochloride, and the method comprises the following steps: carrying out aldehyde group protection on o-nitrobenzaldehyde, and reducing hydrazine hydrate in the presence of a catalyst NiCoB / TiO2 to obtain a compound 3; carrying out bromination reaction on the compound 3 under the action of molecular bromine and hydrogen peroxide, and carrying out deprotection to generate a compound 2; and carrying out aldehyde reductive amination reaction with trans-4-aminocyclohexanol under the action of catalysts NaBH (OAc) 3 and LiClO4, and salifying to obtain the ambroxol hydrochloride. The method has the advantages of mild conditions, simple steps, environmental friendliness, easily stored raw materials and high yield, and is suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD +2
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546458AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationPorphyrinCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
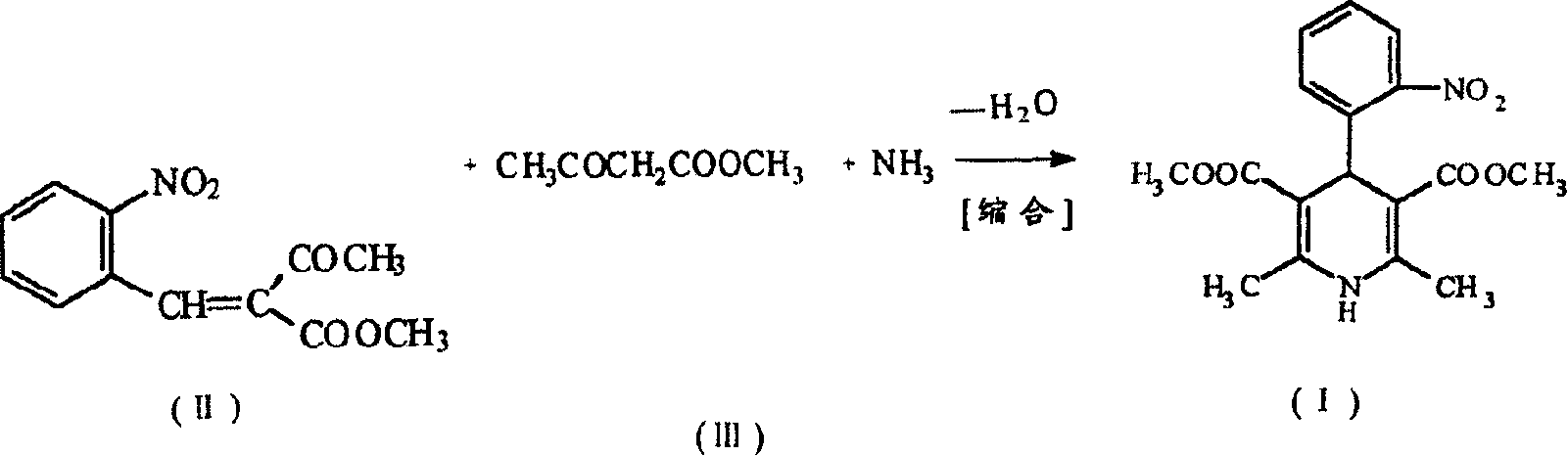
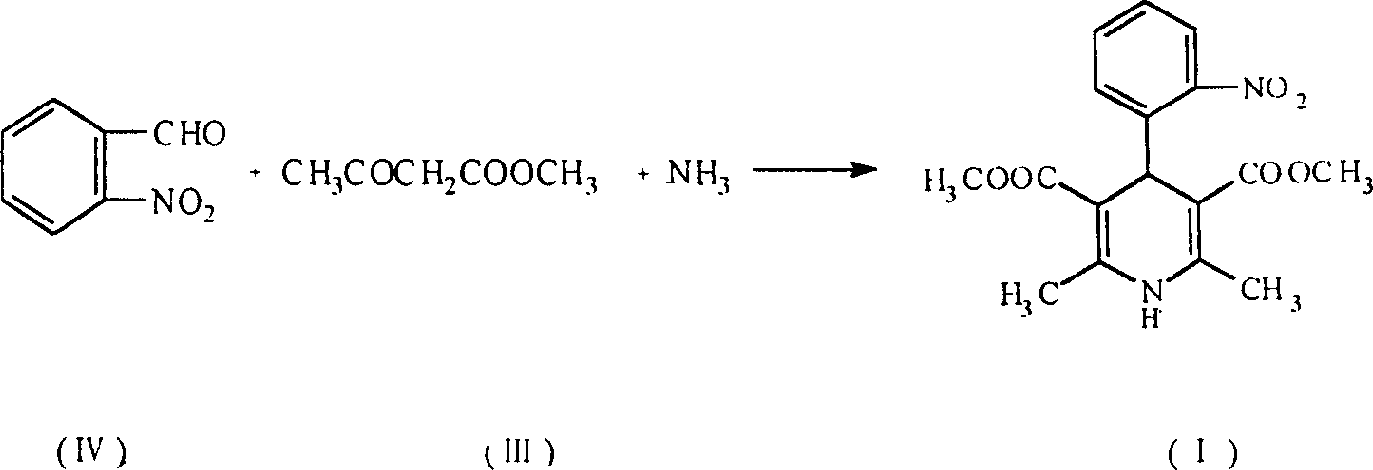
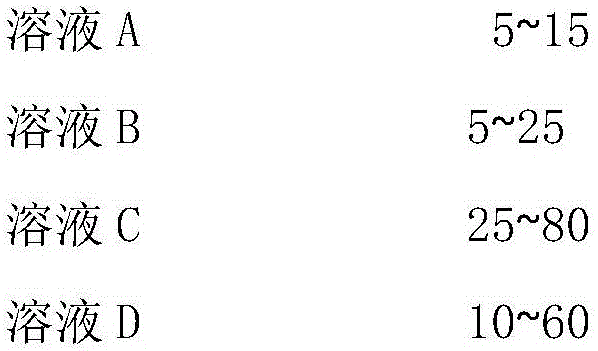
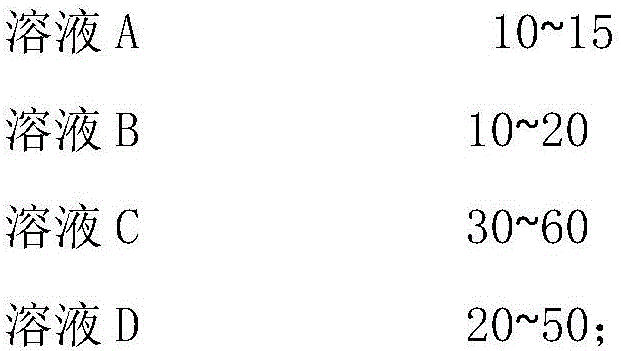

![Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase](https://images-eureka.patsnap.com/patent_img/b7ba7615-59f2-4737-98f0-5eeef335c085/126069DEST_PATH_IMAGE006.PNG)
![Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase](https://images-eureka.patsnap.com/patent_img/b7ba7615-59f2-4737-98f0-5eeef335c085/16981DEST_PATH_IMAGE008.PNG)
![Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase Method for preparing pyrazolo [1, 5-c] quinazoline skeleton compounds by copper catalysis in water phase](https://images-eureka.patsnap.com/patent_img/b7ba7615-59f2-4737-98f0-5eeef335c085/2014102035166100001DEST_PATH_IMAGE001.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/2015100642629100002DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE004.PNG)