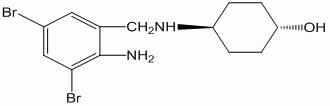
Simple and efficient ambroxol synthesis method
A synthesis method and a technology for ambromine are applied in the field of preparation of known organic compounds, and can solve the problems of difficult control of reaction conditions, reduced yield of side reactions, complicated operations, etc., so as to reduce the generation of organic by-products and improve the yield of products. and purity, the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] At room temperature, in a 100 mL three-neck flask, add 1.5 g of o-nitrobenzaldehyde (10 mmol), 35 mL of 80% ethanol solution (500 mmol), and raise the temperature to 50 °C while stirring, and wait until the o-nitrobenzaldehyde is completely After dissolving, add 2.8 g of reduced iron powder (50 mmol) and 3 drops (1 drop is about 0.05 mL) of concentrated hydrochloric acid, pour out the reaction solution after reacting for 30 min, filter it twice, and add the obtained filtrate to a 100 mL three-necked flask 20 mL of ethanol (350 mmol) was added, and 1.6 g of bromine (10 mmol) was added dropwise with stirring at 20 °C. After 15 min, 3 mL of 30% hydrogen peroxide (100 mmol) was added dropwise. ), after the dropwise addition was completed, reacted for 20 min, after the reaction, the reaction solution was added to 200 mL of saturated sodium bicarbonate solution, fully stirred, and then filtered to obtain the crude product 3,5-dibromo-2-aminobenzaldehyde solid 2.36 g; In a 100...
Embodiment 2
[0023] At room temperature, in a 100 mL three-necked flask, add 1.5 g of o-nitrobenzaldehyde (10 mmol), 38.8 mL of 80% ethanol solution (550 mmol), and raise the temperature to 50 °C while stirring, and wait for the o-nitrobenzaldehyde to After all dissolved, add 3.9 g of reduced iron powder (70 mmol) and 3 drops of concentrated hydrochloric acid, pour out the reaction solution after reacting for 30 min, filter with suction twice, add the obtained filtrate into a 100 mL three-necked flask, and add 28 mL Ethanol (400 mmol), stirring at 20 °C, added dropwise 2.1 g of bromine (13 mmol), 15 min to complete the dropwise addition, and then added dropwise 4.5 mL of 30% hydrogen peroxide (150 mmol), after the dropwise addition was completed, After the reaction was completed, the reaction solution was added to 200 mL of saturated sodium bicarbonate solution, stirred thoroughly, and then filtered to obtain 2.61 g of the crude product 3,5-dibromo-2-aminobenzaldehyde as a solid; in a 100 m...
Embodiment 3
[0025]At room temperature, in a 100 mL three-necked flask, add 1.5 g of o-nitrobenzaldehyde (10 mmol), 42.5 mL of 80% ethanol solution (600 mmol), and raise the temperature to 60 °C while stirring, and wait for the o-nitrobenzaldehyde to After all dissolved, add 5.6 g of reduced iron powder (100 mmol) and 3 drops of concentrated hydrochloric acid, pour out the reaction solution after reacting for 30 min, and after suction filtration twice, add the obtained filtrate into a 100 mL three-necked flask, and then add 28 mL Ethanol (400 mmol), stirring at 20 °C, added dropwise 2.1 g of bromine (13 mmol), 15 min to complete the dropwise addition, and then added dropwise 4.5 mL of 30% hydrogen peroxide (150 mmol), after the dropwise addition was completed, After the reaction was completed, the reaction solution was added to 200 mL of saturated sodium bicarbonate solution and stirred thoroughly, and then filtered to obtain 2.51 g of the crude product 3,5-dibromo-2-aminobenzaldehyde as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com