O-nitrobenzaldehyde and p-nitrobenzaldehyde and preparation method of halides thereof
A technology of p-nitrobenzaldehyde and halogenated substances, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor product quality, serious pollution, difficult to remove, etc., and achieve stable product quality and purity Good, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
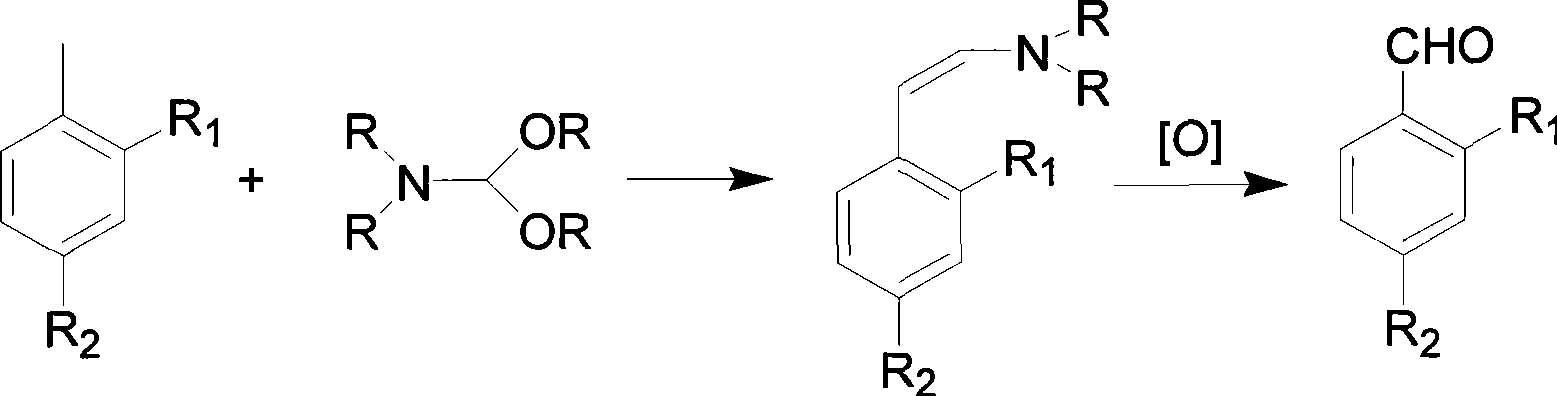
[0026] 1) Preparation of N, N-dimethyl p-nitrostyrylamine
[0027] Add p-nitrotoluene (15.9g, 0.116mol) to N,N-dimethylformamide dimethylformal (23ml, 0.174mol) and N,N-dimethylformamide (101ml), heat to reflux , reacted for 15h, after the reaction was completed, lowered to room temperature to obtain dark red N,N-dimethyl-p-nitrostyrylamine solution, firstly recovered N,N-dimethylformamide diacetal by normal pressure distillation, and then depressurized Recover N,N-dimethylformamide by distillation, drop the raffinate to room temperature, slowly add 100ml of water, stir, and filter with suction to obtain N,N-dimethyl-p-nitrostyrylamine, which is directly used in the next step without purification reaction.
[0028] 2) preparation of p-nitrobenzaldehyde
[0029] With tetraethylammonium bromide (0.64g), the N,N-dimethyl-p-nitrostyrylamine obtained in the previous step was dissolved in a mixed solution of 200ml water and 100ml DMF, cooled with ice water, and slowly added dropwi...
Embodiment 2
[0031] 1) Preparation of N, N-dimethyl-2-chloro-4-nitrostyrylamine
[0032] 2-Chloro-4-nitrotoluene (19.9g, 0.116mol) was added to N,N-dimethylformamide dimethylformal (46ml, 0.348mol) and N,N-dimethylformamide (105.4ml ), heated to reflux for 14 hours, the reaction was completed, and cooled to room temperature to obtain a magenta N, N-dimethyl-2-chloro-4-nitrostyrylamine solution, which was first recovered by atmospheric distillation to recover N, N- Methylformamide dimethylformyl, then distilled under reduced pressure to recover N,N-dimethylformamide, the raffinate was lowered to room temperature, slowly added 100ml of water, stirred, and suction filtered to obtain purple N,N-dimethyl-2- Chloro-4-nitrostyrylamine was directly used in the next step without purification.
[0033] 2) Preparation of 2-chloro-4-nitrobenzaldehyde
[0034] Dissolve tetrabutylammonium chloride (0.5g), N, N-dimethyl-2-chloro-4-nitrostyrylamine prepared in the previous step in a mixed solution of 18...
Embodiment 3
[0036] 1) Preparation of N, N-dimethyl-2-bromo-4-nitrostyrylamine
[0037]2-Bromo-4-nitrotoluene (25.1g, 0.116mol) was added to N,N-dimethylformamide dimethylformal (30.7ml, 0.232mol) and N,N-dimethylformamide (106.4 ml), heated to reflux for 14 hours, the reaction was completed, and cooled to room temperature to obtain a purple-red N, N-dimethyl-2-bromo-4-nitrostyrylamine solution, which was first recovered by atmospheric distillation to recover N, N- Dimethyl formamide dimethyl acetal, then distilled under reduced pressure to recover N,N-dimethylformamide, the raffinate was lowered to room temperature, slowly added 100ml of water, stirred, and suction filtered to obtain purple N,N-dimethyl-2 -Bromo-4-nitrostyrylamine, without refining, is directly used in the next step reaction.
[0038] 2) Preparation of 2-bromo-4-nitrobenzaldehyde
[0039] Benzyltriethylammonium bromide (0.5g), N, N-dimethyl-2-bromo-4-nitrostyrylamine obtained in the previous step were dissolved in the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com