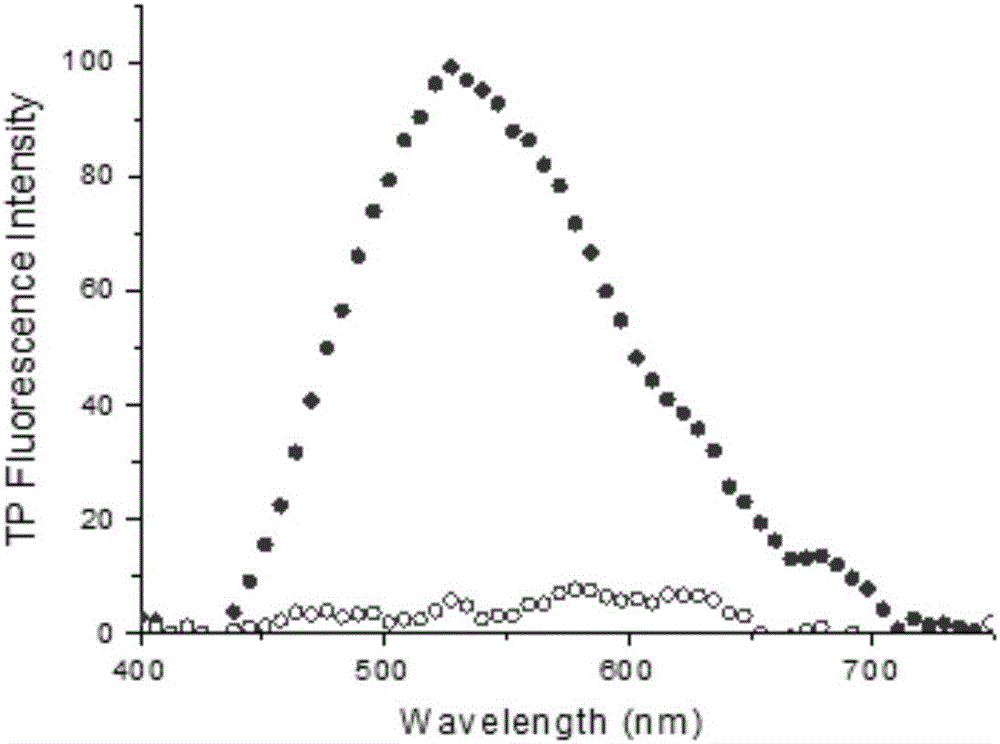
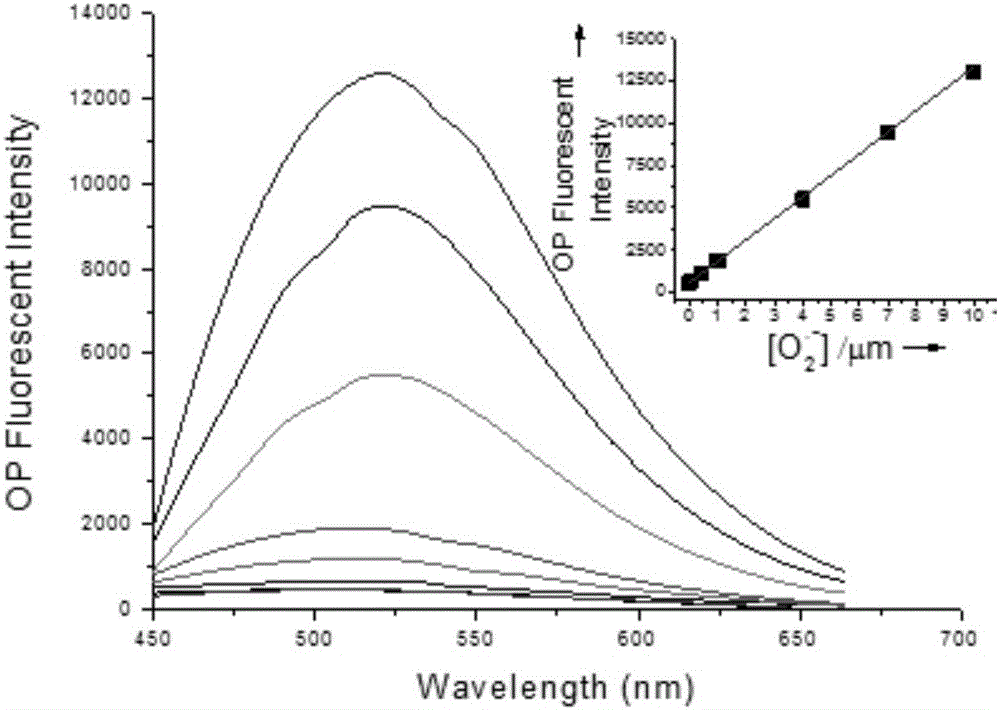
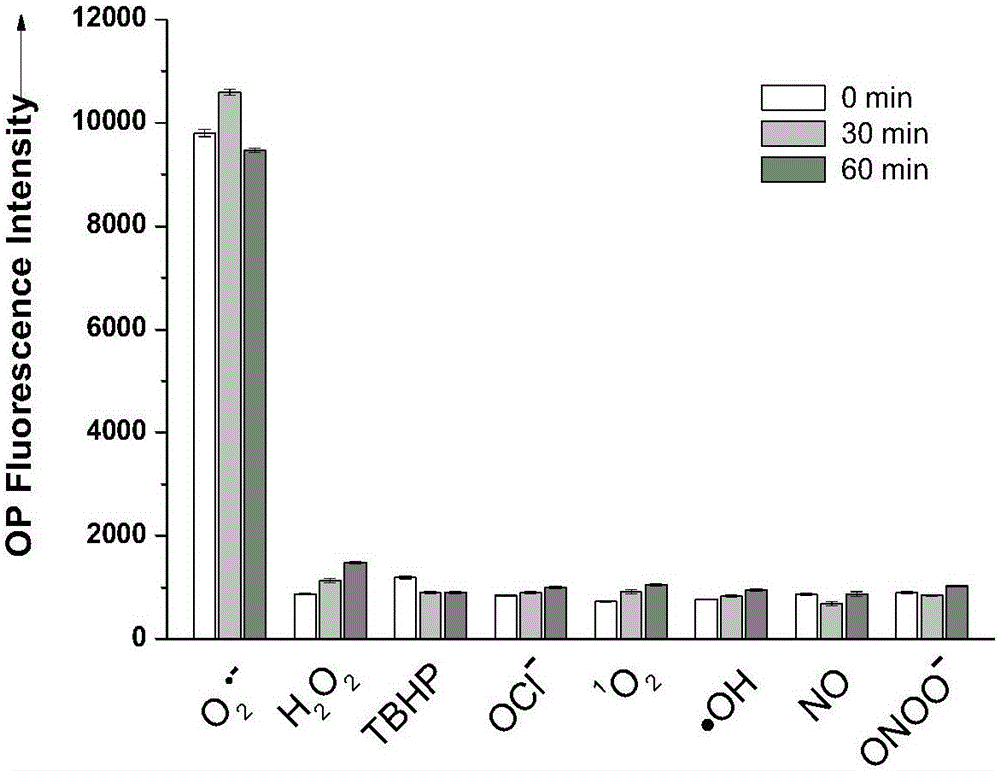
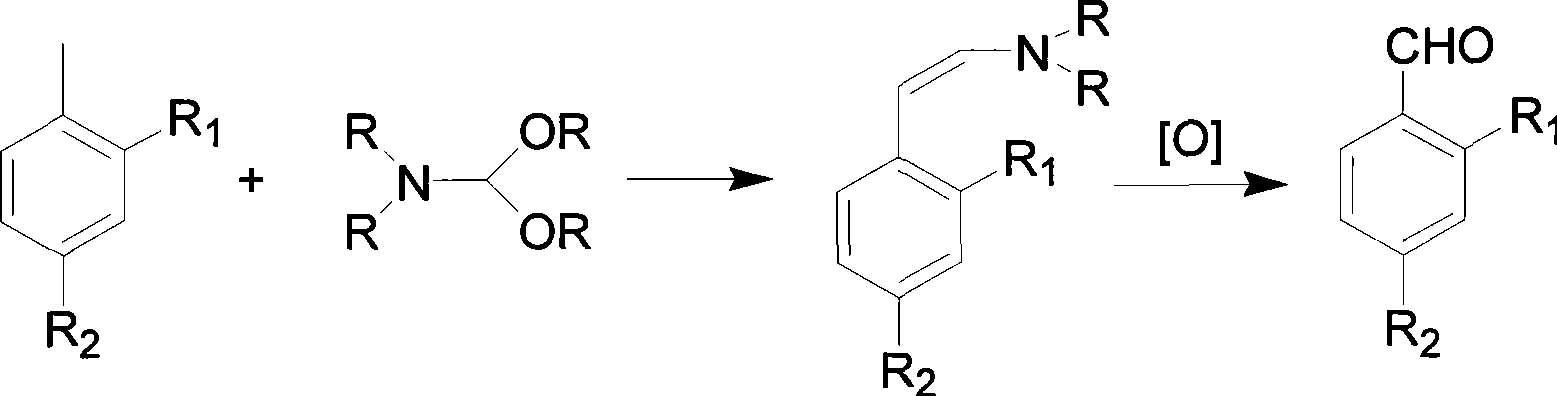
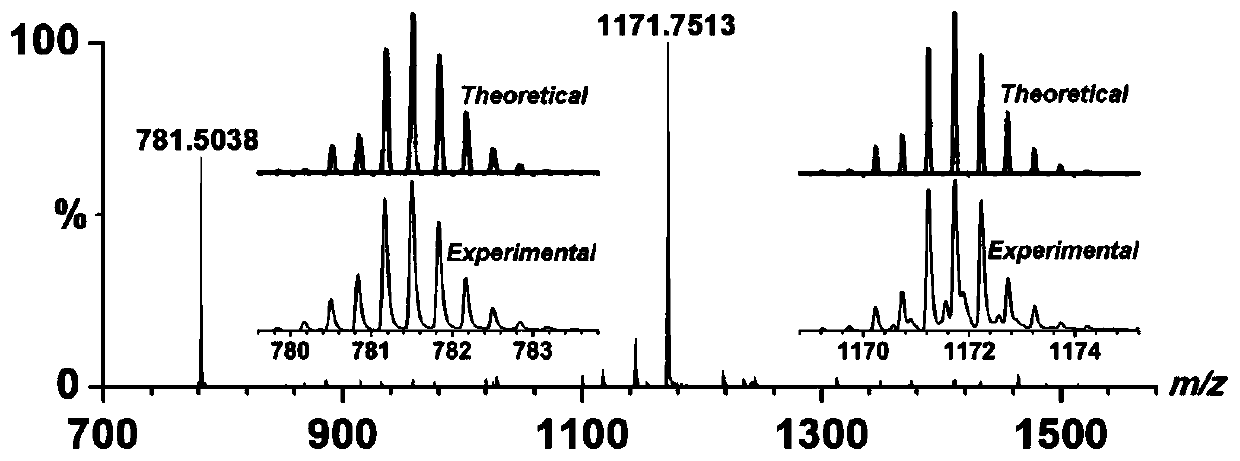
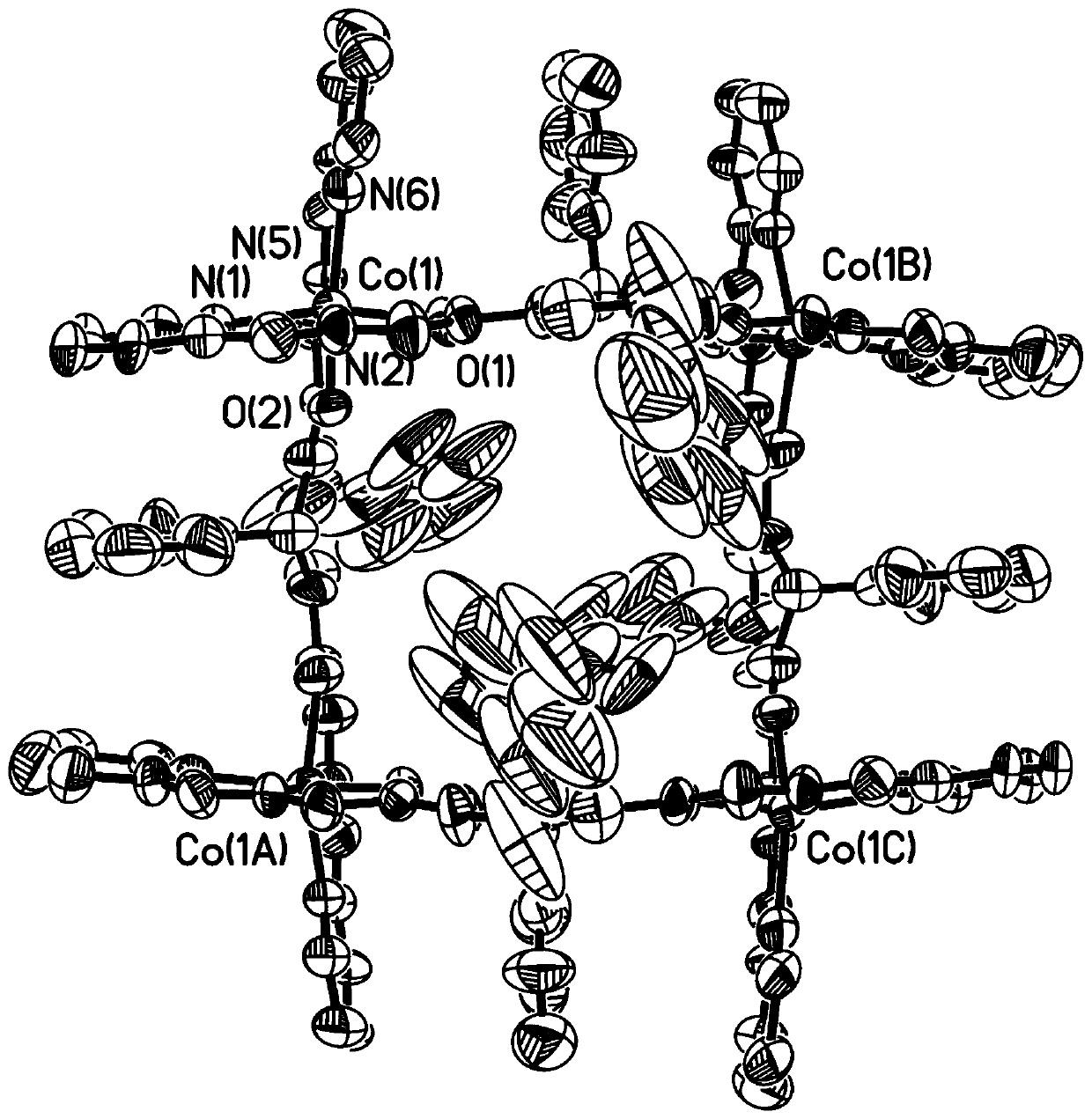
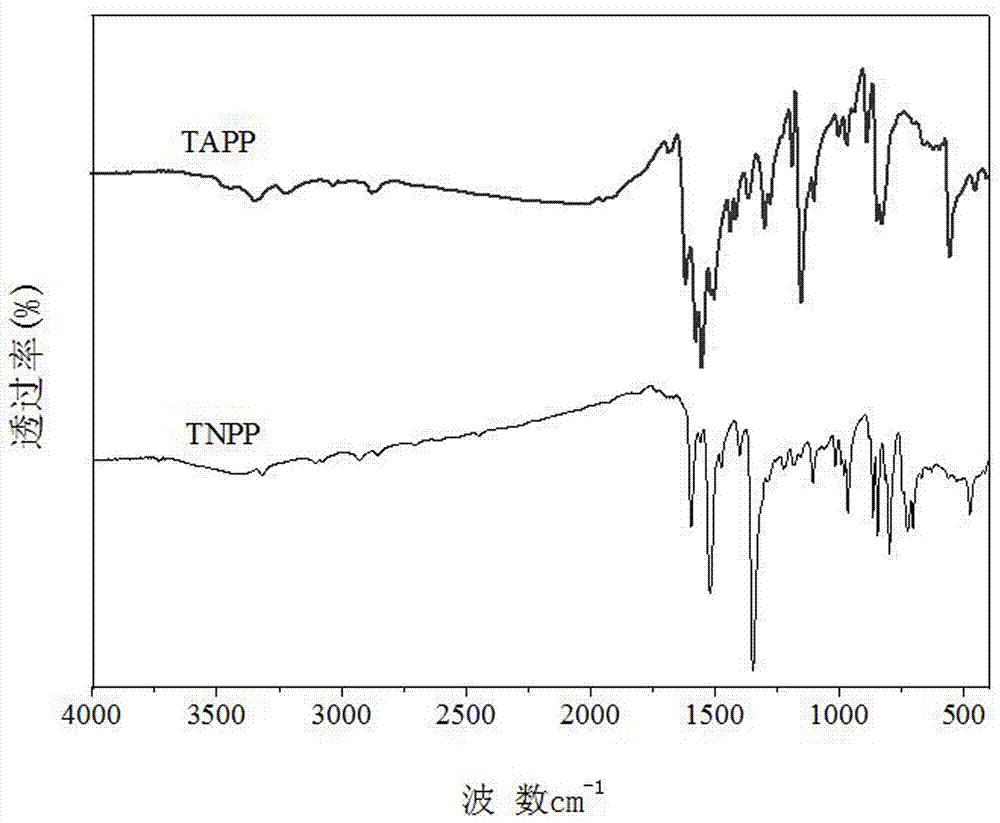
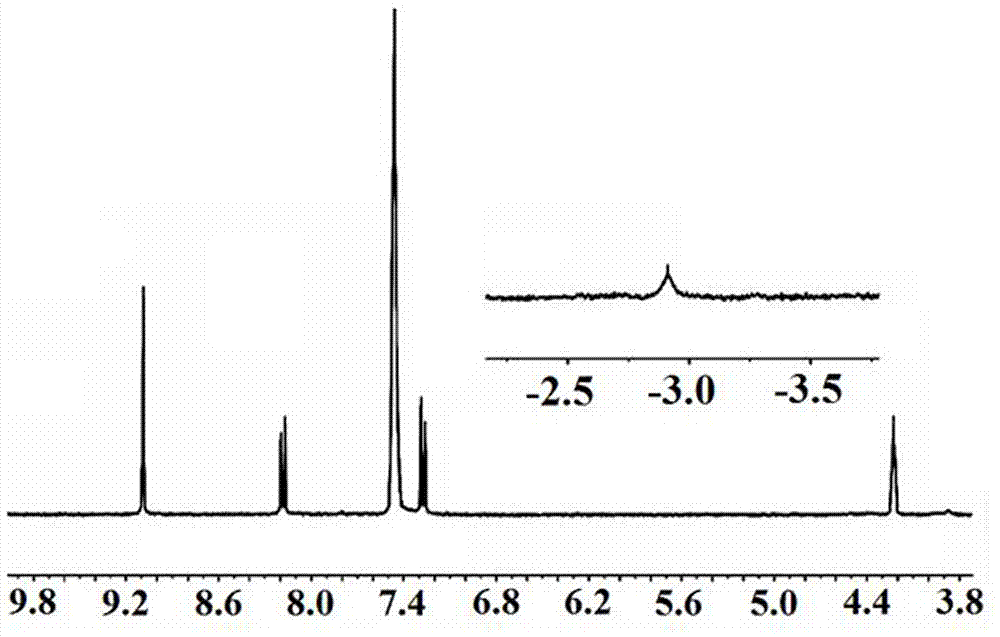
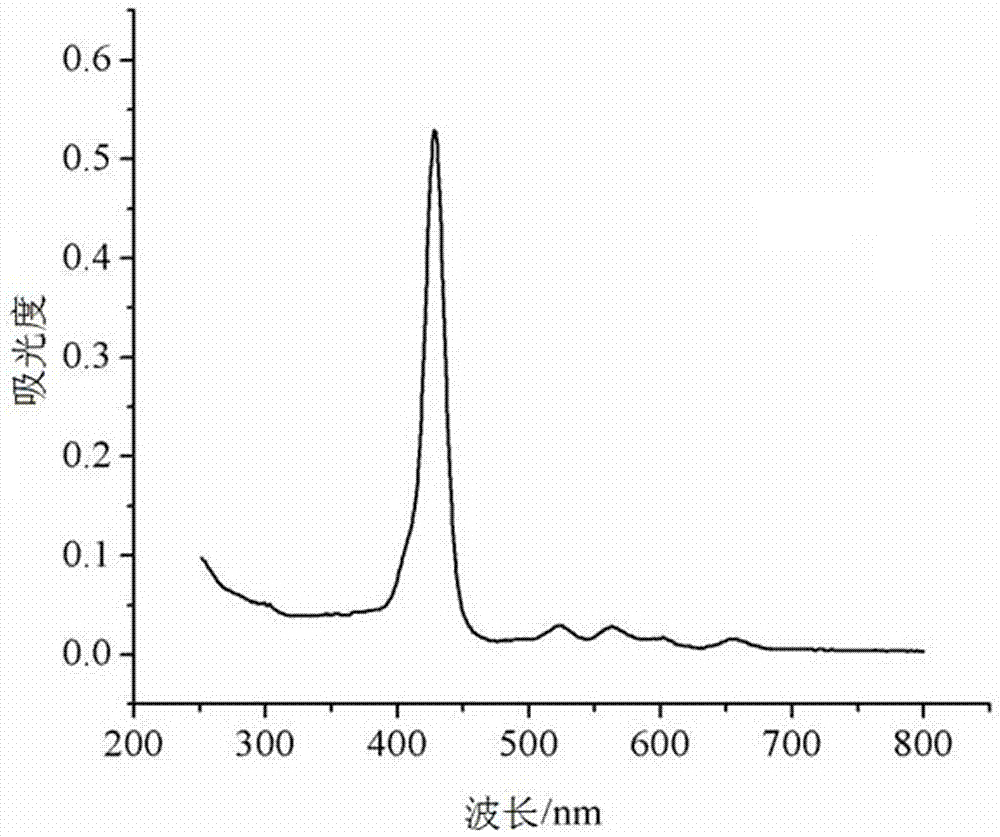
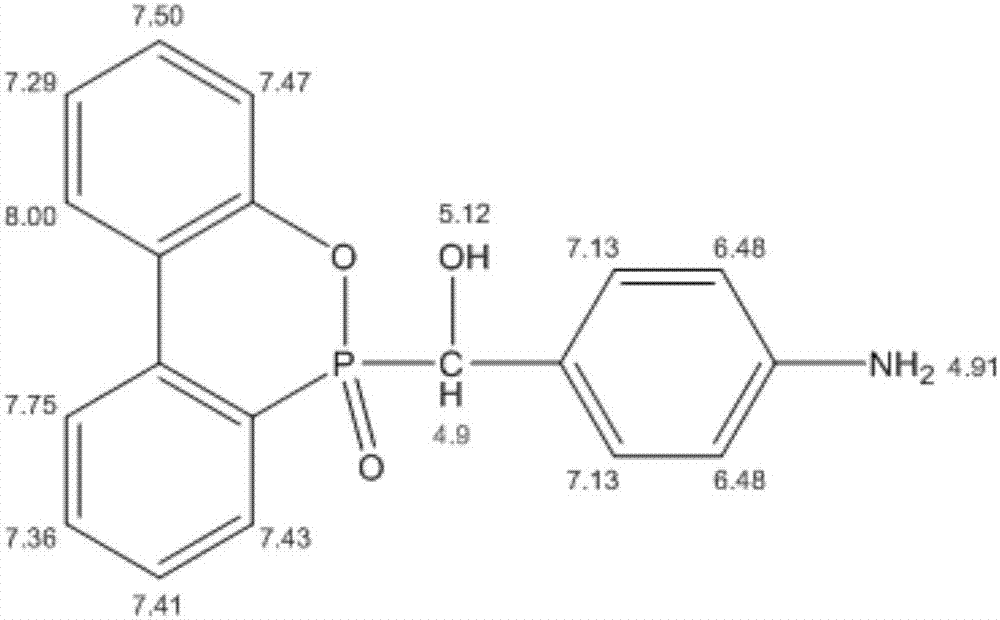
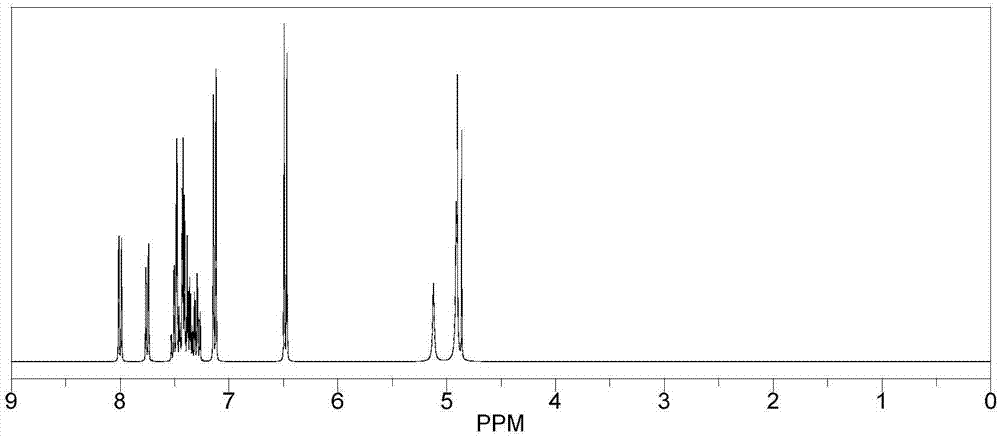
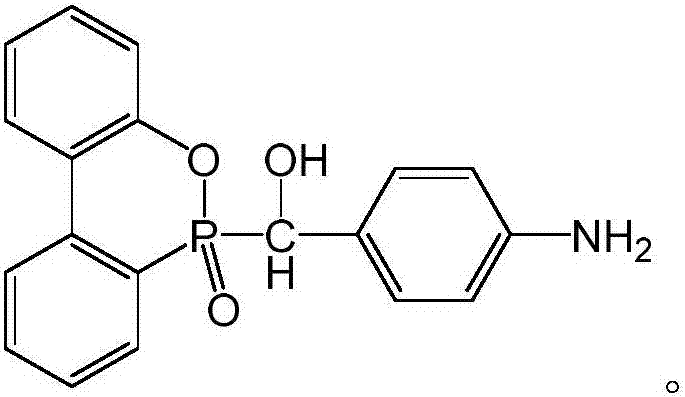
Patents
Literature
61 results about "Para-nitrobenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluorescent probe as well as preparation method and application thereof
InactiveCN105154071AGood chemical stabilityGood photophysical stabilityOrganic chemistryFluorescence/phosphorescenceFluorescenceCaffeic acid
The invention discloses a fluorescent probe as well as a preparation method and application thereof. A structural formula of the fluorescent probe is shown in a formula I in the specification. The preparation method of the fluorescent probe comprises the following steps: mixing p-nitrobenzaldehyde, 2,5-dimethylpyrazine and a catalyst to obtain mixed liquor and heating the mixed liquor to 150-180 DEG C to react, thus obtaining PY-NO2; adding sodium polysulfide to PY-NO2 to react to obtain PY-NH2; adding PY-NH2, caffeic acid, triethylamine, HOBT and EDC.HCL to a mixed solvent of dichloromethane and DMF to obtain mixed liquor; and stirring the mixed liquor under the protection of an inert gas to obtain a crude product of a two-photon fluorescent probe, and purifying the crude product to obtain a pure product of the two-photon fluorescent probe. The fluorescent probe has good chemical stability and photo-physical stability, can sensitively respond to O2<.-> in acidic, neutral and alkaline ranges, has higher measurement sensitivity and is very short in response time.
Owner:SHANDONG NORMAL UNIV
O-nitrobenzaldehyde and p-nitrobenzaldehyde and preparation method of halides thereof
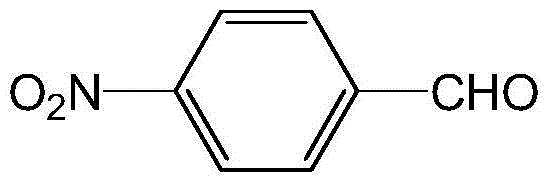
InactiveCN101362697AEasy to operateMild responseOrganic chemistryOrganic compound preparationOrtho positionPara-nitrobenzaldehyde
The invention discloses a preparation method for o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof, which comprises the following steps in sequence: toluene is used as raw material, wherein, ortho-position and para-position of toluene contain nitryl or nitryl and halogen. N,N-dialkyl formamide is used as solvent, and the raw material and the solvent are condensed with N,N-dialkyl formamide tirethylene ethanol to prepare N,N-dialkyl styrylamine, wherein, ortho-position and para-position of N,N-dialkyl styrylamine contain nitryl or nitryl and halogen; then, with the existence of phase transfer catalyst, the N,N-dialkyl styrylamine prepared is oxidized by taking peroxoic acid or hydrogen peroxide as oxidizer to produce o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof. By adopting the preparation method of the invention to prepare o-nitrobenzaldehyde, p-nitrobenzaldehyde and the phenylhalostannyl thereof, the operation is simple, the reaction conditions are easy to control, the raw material is easily available, the yield is higher, the product quality is stable and the purity is good.
Owner:ZHEJIANG UNIV
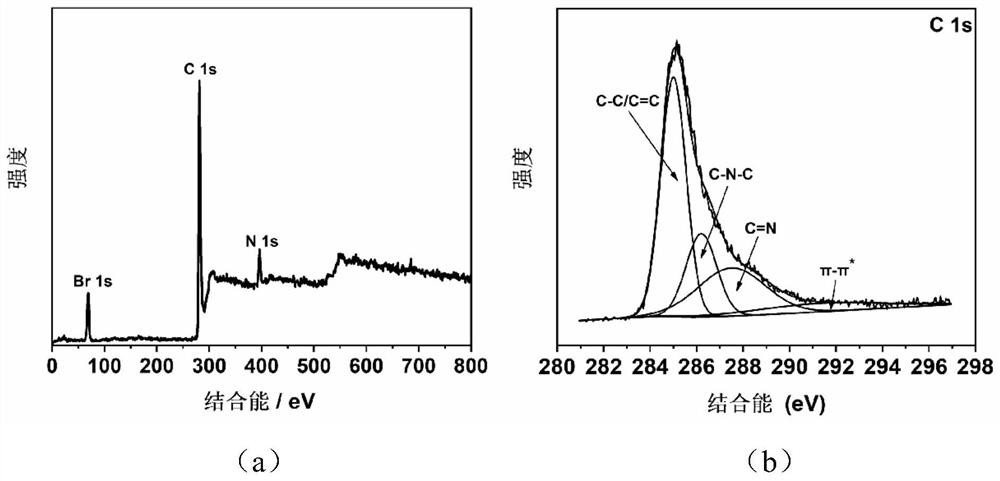
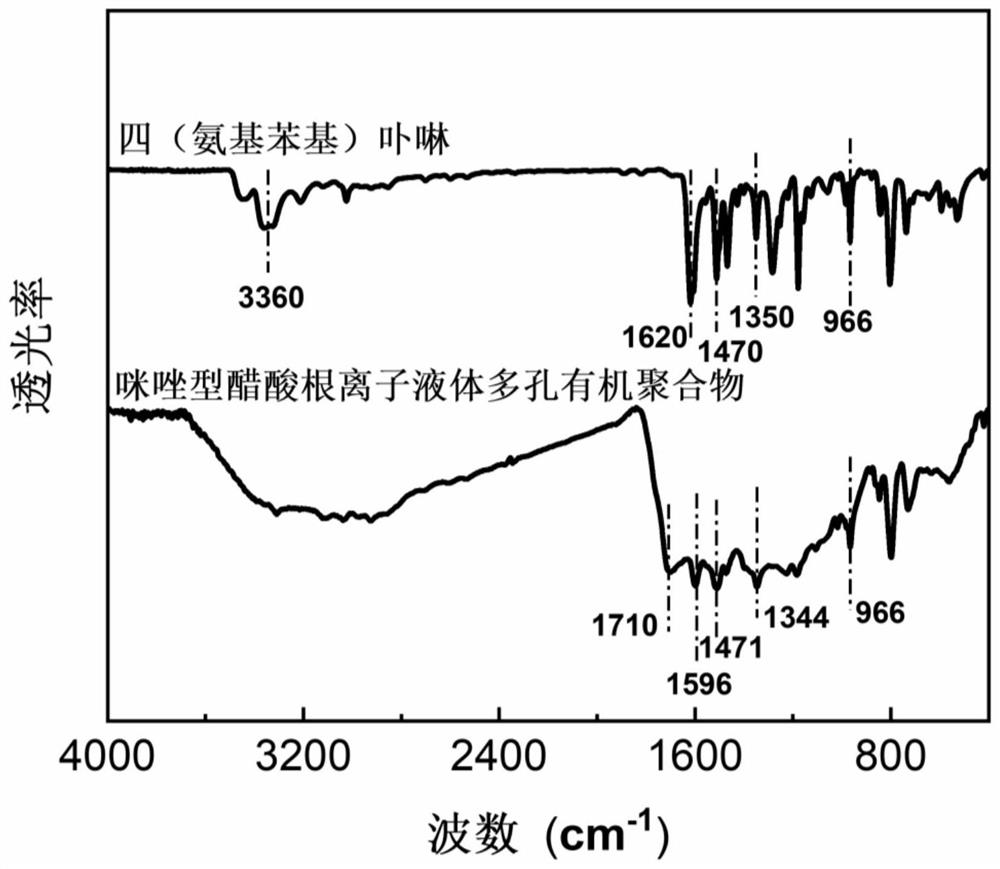
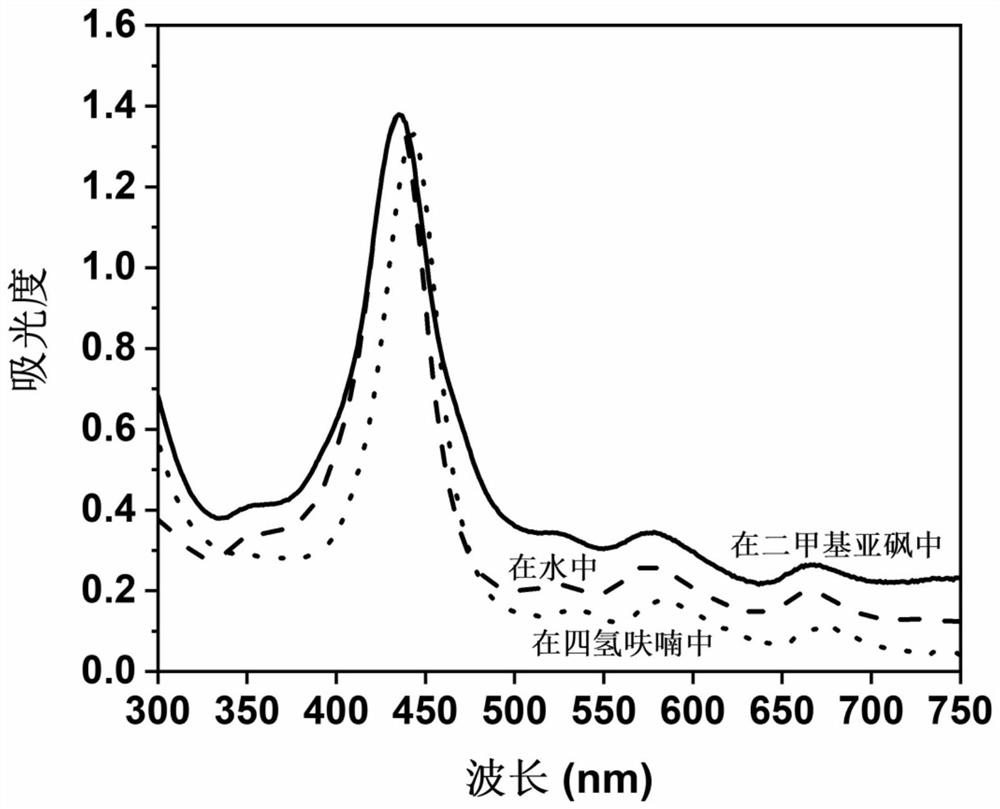
Preparation method of imidazole type ionic liquid porous organic polymer
InactiveCN112321824ASolve pollutionSolve the costOrganic-compounds/hydrides/coordination-complexes catalystsPolymer scienceAcetic anhydride
The invention belongs to the technical field of polymer synthesis, and discloses a preparation method of an imidazole type ionic liquid porous organic polymer. The method comprises the following steps: synthesizing tetra(nitrophenyl)porphyrin by using p-nitrobenzaldehyde and pyrrole as monomers and propionic acid and acetic anhydride as solvents through a propionic acid-anhydride method; reducingthe tetra(nitrophenyl)porphyrin by using a reducing agent to obtain tetra(aminophenyl)porphyrin; and enabling amino groups to form imidazole ring cations with a 1,2-dicarbonyl compound and an alkylaldehyde compound under an acidic condition, and allowing porphyrin monomers to connected with one another through imidazole rings, thereby forming the heterogeneous porous organic polymer. According tothe preparation method, polymerization can be realized by virtue of Debuus-Radziszewski imidazole synthesis reaction of substituted anilino functional groups on four mutually symmetrical methylenes oftetra(aminophenyl)porphyrin, so the ionic liquid porous organic polymer containing imidazole type cation / anion groups is obtained, and the preparation of a non-metal heterogeneous photocatalyst can be greatly simplified.
Owner:HUAZHONG UNIV OF SCI & TECH
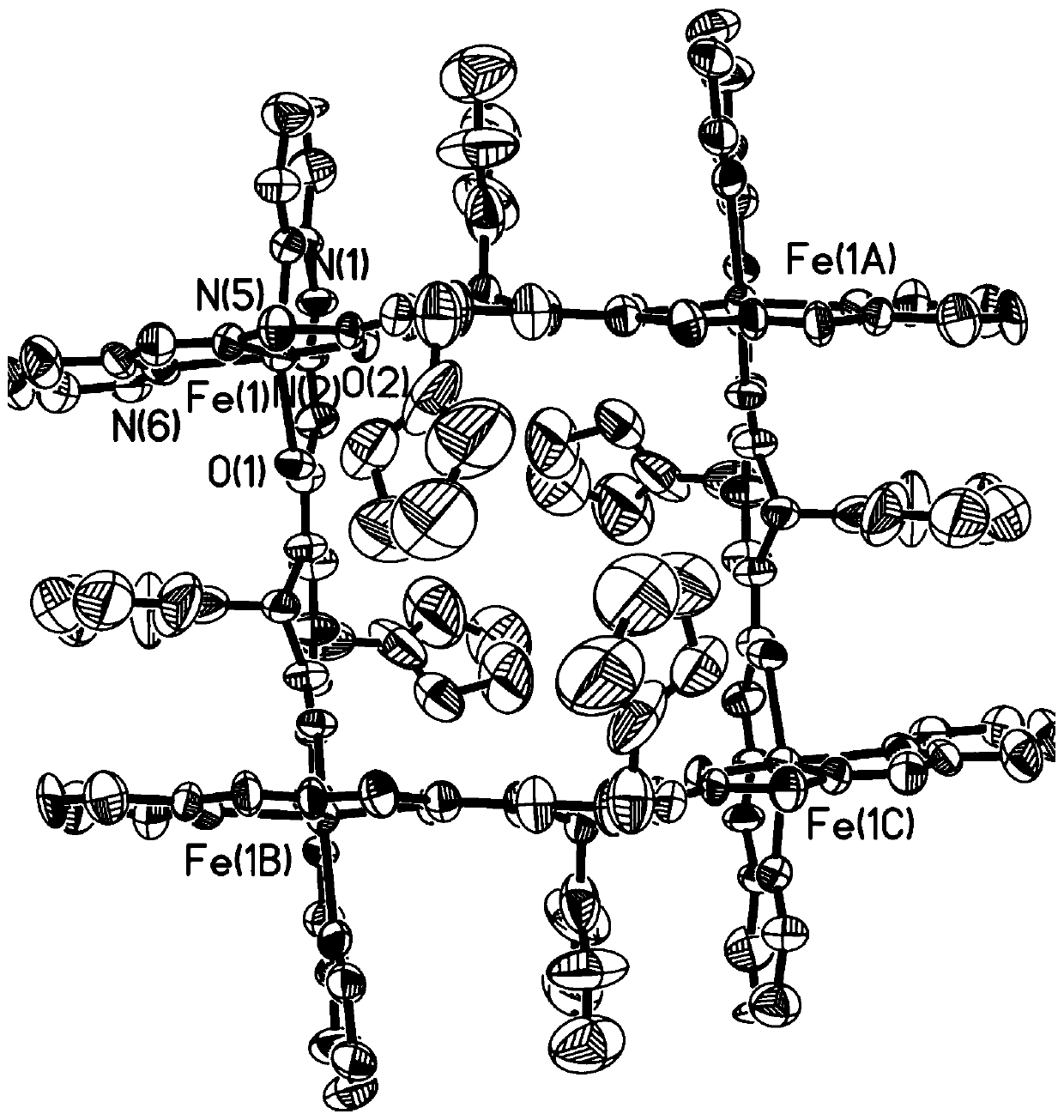
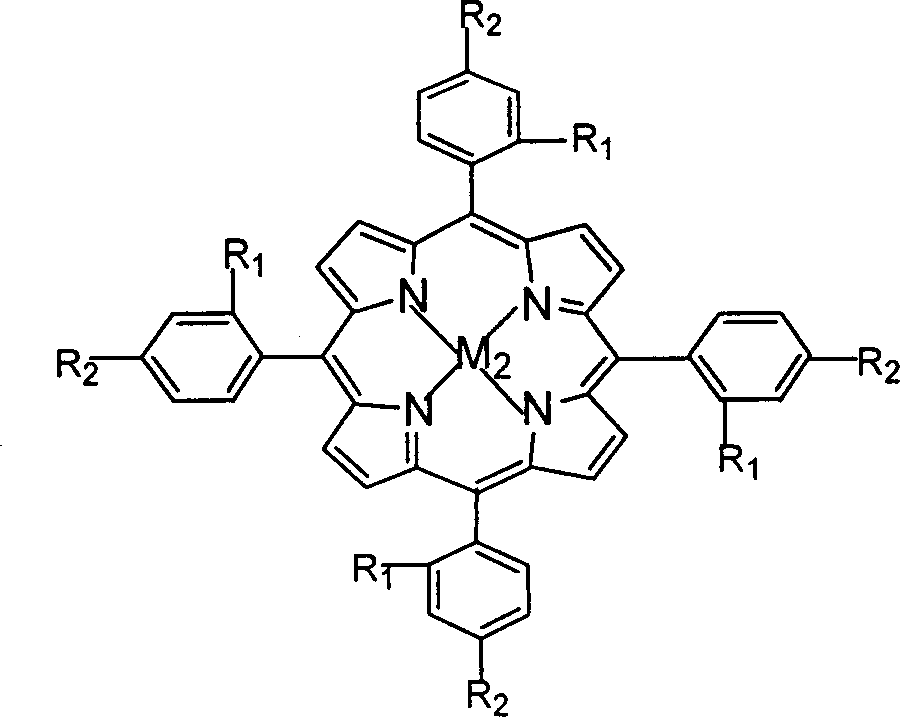
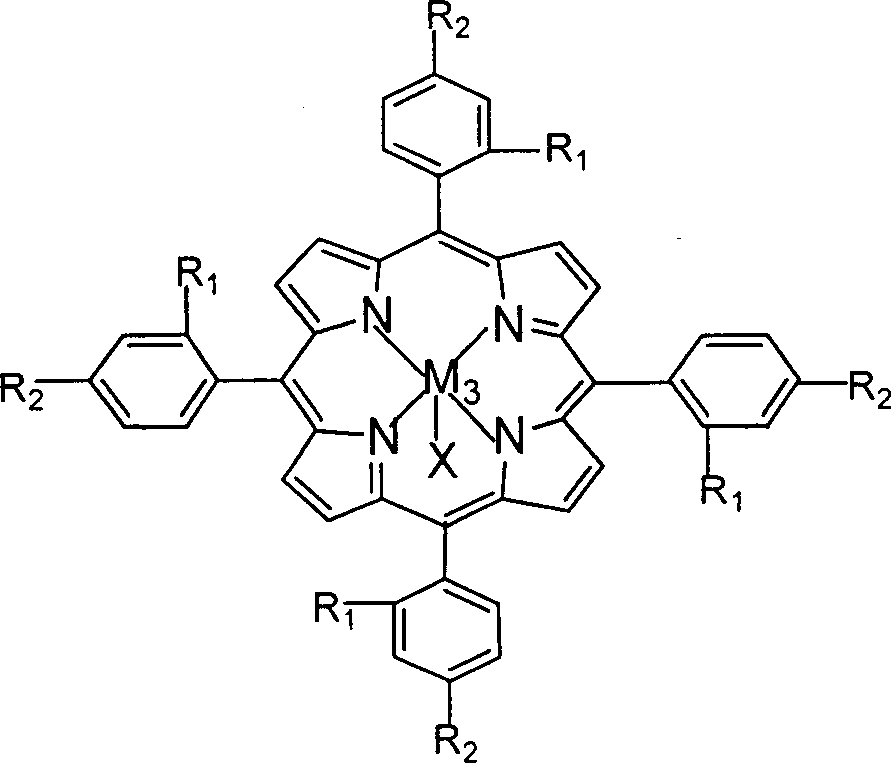
Preparation method and application of adjustable metal organic cage compound for efficiently selective catalytic reduction of nitrobenzaldehyde
InactiveCN110483585AHigh yieldChemically stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroboratePara-nitrobenzaldehyde
The invention belongs to the technical field of fine chemical engineering. The invention relates to a preparation method and application of an adjustable metal organic cage compound for efficient selective catalytic reduction of nitrobenzaldehyde. According to the preparation method, M<2+> in a transition metal salt is used as a node and L is used as a ligand for reaction to prepare the metal organic cage compound, and the synthetic route is as follows: M<2+> + L- to M-L; wherein the ligand L is selected from H2FPB; the transition metal salt is selected from one of ferrous perchlorate, cobalttetrafluoroborate, nickel perchlorate or zinc tetrafluoroborate. The metal organic cage compound prepared by the method is low in raw material price and high in yield, and the obtained compound is stable in chemical property and easy to put into practical application. As a target compound M-FPB, the adjustable metal organic cage compound shows that the selectivity of the compound M-FPB can reach 99% in the aspects of reduction of p-nitrobenzaldehyde to prepare p-nitrobenzyl alcohol, one-step synthesis of cinnarizine by reduction catalysis of cinnamyl aldehyde and reduction of p-nitrobenzaldehyde to prepare p-aminobenzaldehyde.
Owner:DALIAN UNIV OF TECH
Fireproof high-temperature-resistant building outer wall board
InactiveCN105907036AGood fire resistance and heat resistanceImprove fire resistance and heat resistanceBuilding componentsInsulation layerWear resistant
The invention discloses a fireproof high-temperature-resistant building outer wall board, which comprises a base layer, a heat insulation layer, a surface mortar layer, a fireproof high-temperature-resistant layer, a waterproof layer and a decoration wear-resistant coating in sequential arrangement from inside to outside, wherein the fireproof high-temperature-resistant layer is prepared from the following raw materials in parts by weight: 109 to 112 parts of modified phenolic resin, 25 to 28 parts of alkyd resin, 22 to 25 parts of ethylene-ethylic acid ethylene copolymer, 13 to 16 parts of polyether sulfone, 0.7 to 1.0 part of silane coupling agents, 18 to 21 parts of polyacrylonitrile fiber, 22 to 25 parts of ramie, 22 to 25 parts of kieselguhr, 14 to 17 parts of nanosilicon dioxide, 33 to 36 parts of ground limestone, 16 to 19 parts of melamine pyrophosphate and 2 to 3 parts of anti-aging agents. A preparation method of the modified phenolic resin comprises the following steps that hydroquinone and diphenyl chlorophosphate take a reaction; then, p-nitrobenzaldehyde is added for reaction; boracic acid and acidized ammonium molybdate are added for reaction to obtain modified phenolic resin.
Owner:ANHUI DEQUAN NEW BUILDING MATERIALS TECH CO LTD
Method for synthesizing TNPP and TAPP at high yield and high efficiency
InactiveCN107056793AHigh purityIncrease reaction rateOrganic chemistryChemical synthesisPropionic anhydride
The invention provides a method for synthesizing TNPP and TAPP at high yield and high efficiency and belongs to the technical field of chemical synthesis. The method provided by the invention is characterized in that a small amount of acetic anhydride or propionic anhydride is added to serve as a catalyst in the reaction process of nitrobenzaldehyde and pyrrole, so that the reaction speed is effectively improved and the reaction is performed rightwards to the maximum extent; hot pyrrole is adopted for dissolving and purifying the TNPP to obtain a TNPP product with high purity; in the process of synthesizing the TAPP, a strong reducer tin chloride is adopted for ammonifying the TNPP, and trichloromethane capable of dissolving the TAPP is adopted for efficiently separating the TAPP from an organic phase by adopting a Soxhlet extraction method, so that the purity and the yield of the TAPP are effectively improved.
Owner:NORTHWEST NORMAL UNIVERSITY
Novel DOPO-type epoxy resin curing agent and preparation method of same
ActiveCN107501526APlay a role in cross-linking and curingAchieve halogen-free flame retardant effectGroup 5/15 element organic compoundsEpoxyIron powder
The invention relates to a novel compound, 10-((4-aminophenyl)(hydroxyl)methyl)-9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, as well as a preparation method of same. The compound is prepared by performing a reaction to raw materials, including DOPO and p-nitrobenzaldehyde, in ethanol or a DMF solvent to obtain an intermediate product, and then reducing the intermediate product with iron powder, and finally neutralizing and purifying the product to obtain the compound. The compound can be used as a curing agent and a flame retardant for epoxy resin. The preparation method employs easy-to-obtained raw materials and has simple processes, so that the compound is convenient to promote and apply.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
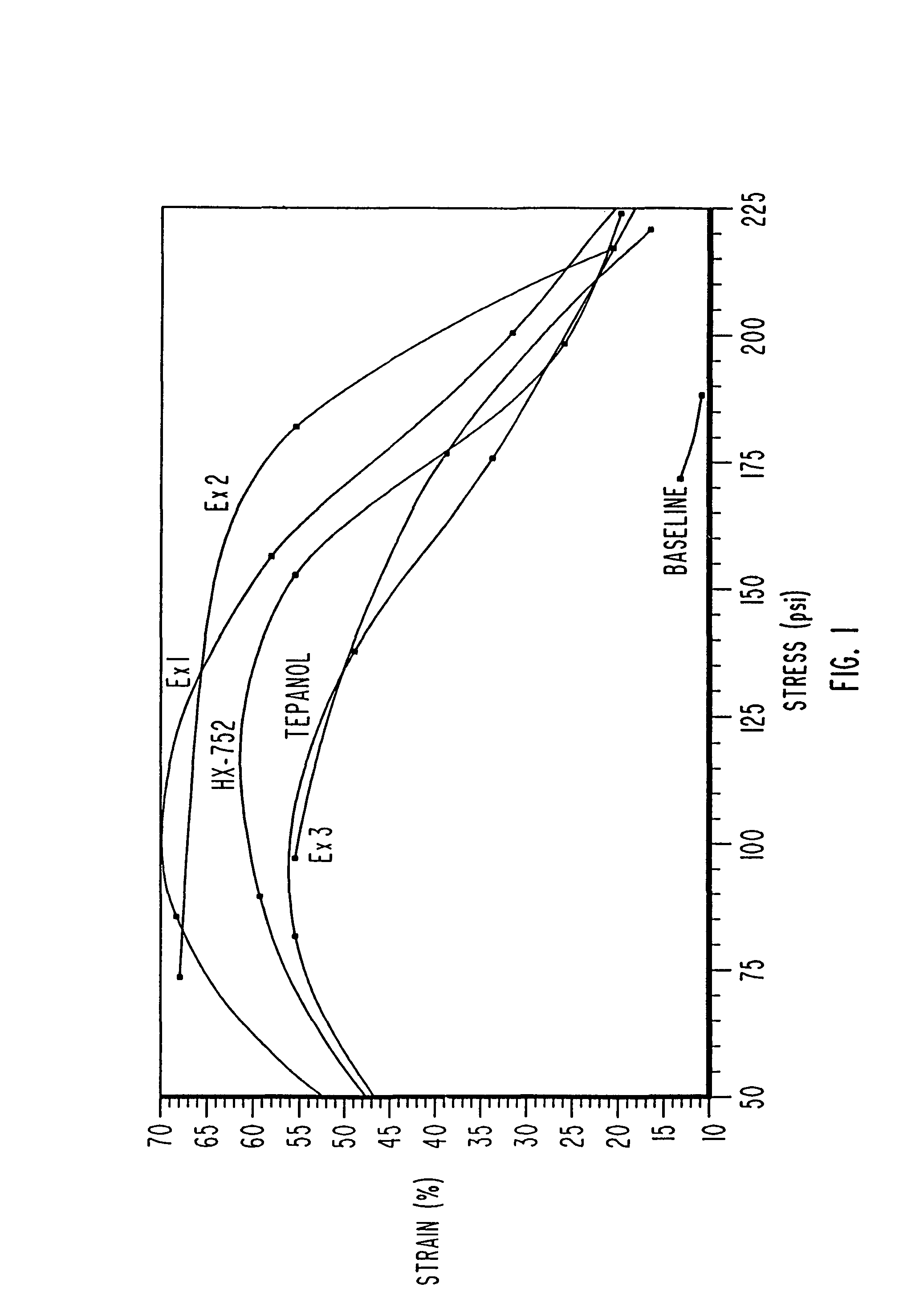
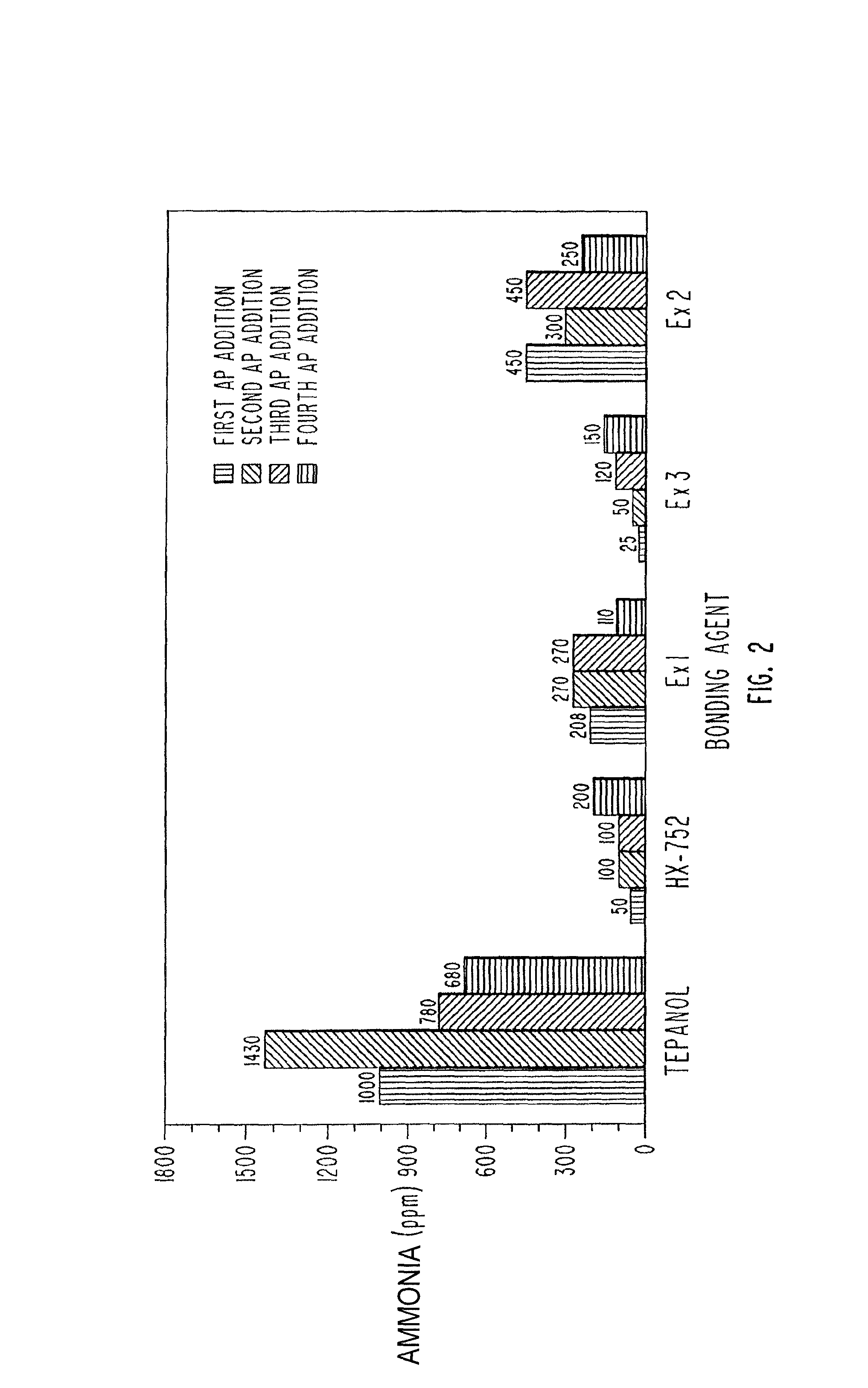
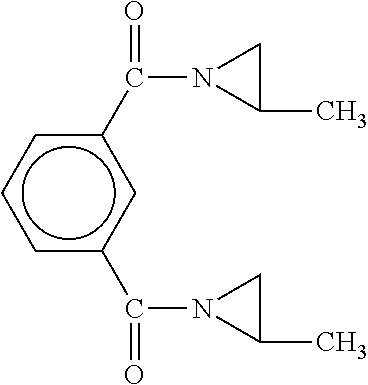
Solid propellant bonding agents and methods for their use
ActiveUS9181140B1Avoid crackingEffective combinationNon-explosive/non-thermic compositionsAmmonium perchlorate explosive compositionsAmmonia productionMachining process
Bonding agents for use in formulating solid propellants are described. The bonding agents have a Schiff base, or Schiff base and hydroxyl or amine functionality. These materials are synthesized by reacting a primary amine with an aldehyde to form a Schiff base. By definition, a Schiff base comprises the reaction product of a primary amine and an aldehyde. In one embodiment of the invention, such a product is produced by the reaction of a polyoxyalkyleneamine (JEFFAMINE®) with p-nitrobenzaldehyde and glycidol. The result is a polyether having both hydroxyl and Schiff base functionality. The bonding agents provide superior performance, while avoiding problems, such as ammonia production during processing, encountered when using existing bonding agents. Propellants formulated using these bonding agents have mechanical properties within acceptable ranges.
Owner:NORTHROP GRUMMAN INNOVATION SYST INC
Preparation method of p-nitrobenzaldehyde
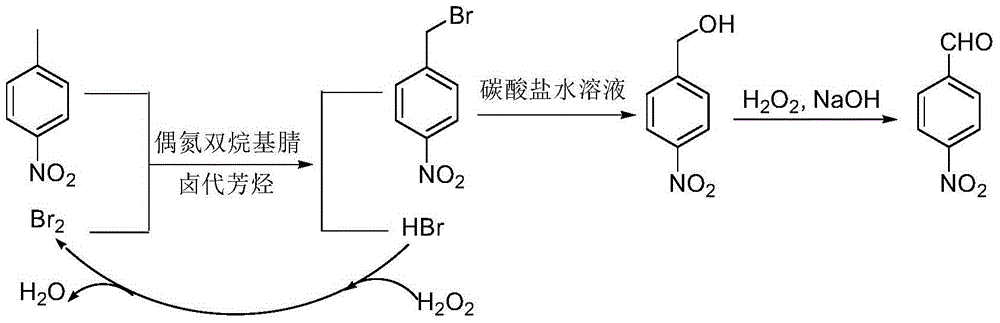
InactiveCN105348107AEasy to cleanReduce pollutionOrganic chemistryOrganic compound preparationP-nitrotolueneSolvent
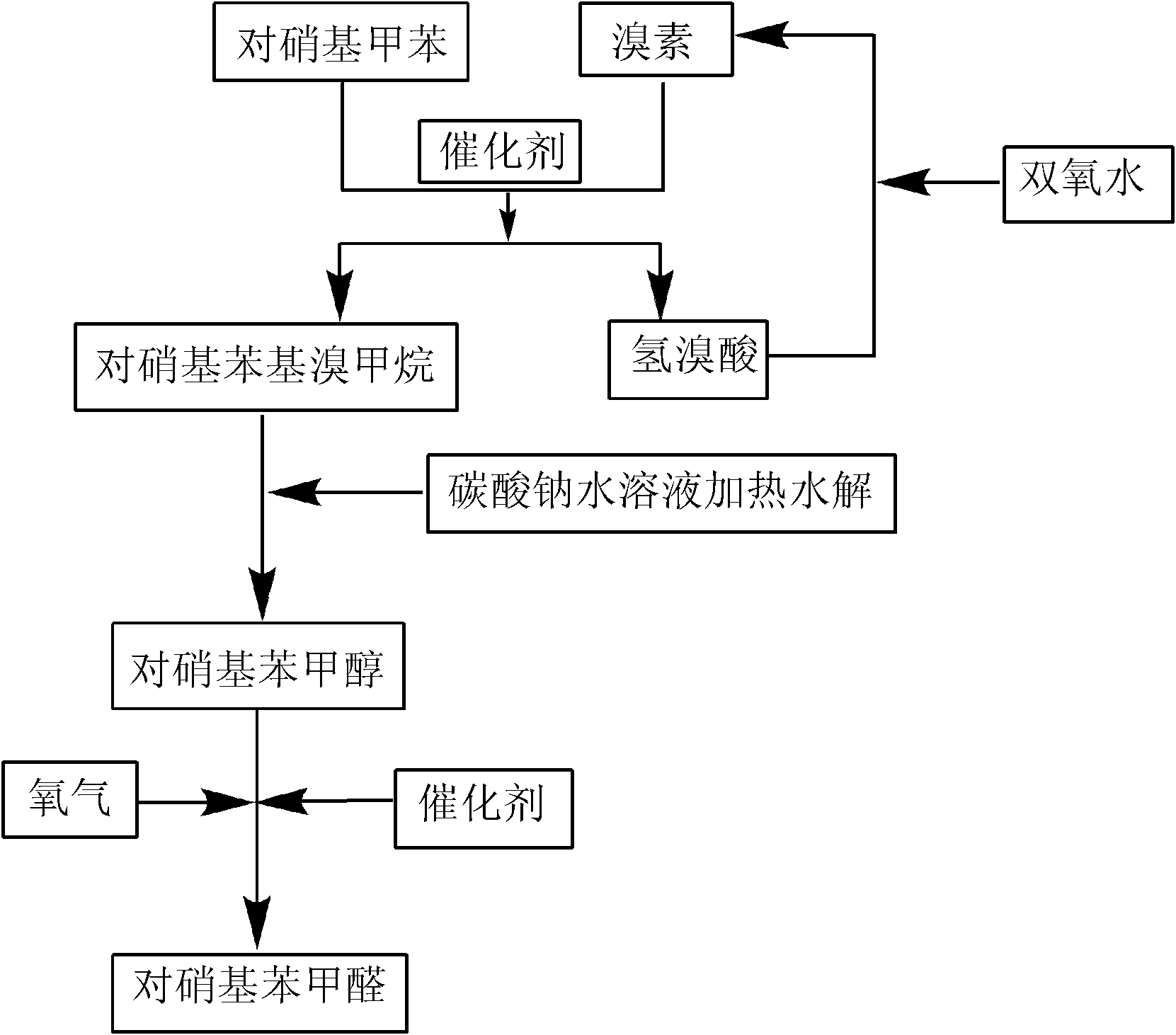
The invention discloses a preparation method of p-nitrobenzaldehyde. The preparation method comprises the following steps: p-nitrotoluene, which is used as a raw material, undergoes bromine bromination under the catalysis of azo-dialkyl nitrile, so as to generate 4-nitrobenzyl bromide and hydrogen bromide; hydrolysis of 4-nitrobenzyl bromide is catalyzed by an aqueous carbonate solution to obtain p-nitrobenzyl alcohol; and hydrogen peroxide oxidation of p-nitrobenzyl alcohol is catalyzed by sodium hydroxide so as to generate a target product, namely p-nitrobenzaldehyde. According to the invention, the azo-dialkyl nitrile solid catalyst replaces a peroxycarbonate liquid phase catalyst to catalyze the bromination reaction, so as to raise operational safety of the industrial preparation reaction; by using aryl halide as a solvent medium, use of a haloalkane solvent medium is avoided, and pollution of volatile organic solvents with low boiling point is avoided; and by the hydrogen peroxide oxidation method, cleanliness of the industrial preparation reaction is raised, and environmental pollution is reduced. According to the invention, product yield is increased; yield is raised by about 3% in comparison with yield of existing traditional industrial methods; overall yield reaches 76%; and product purity reaches 99% and above.
Owner:NANJING UNIV OF SCI & TECH
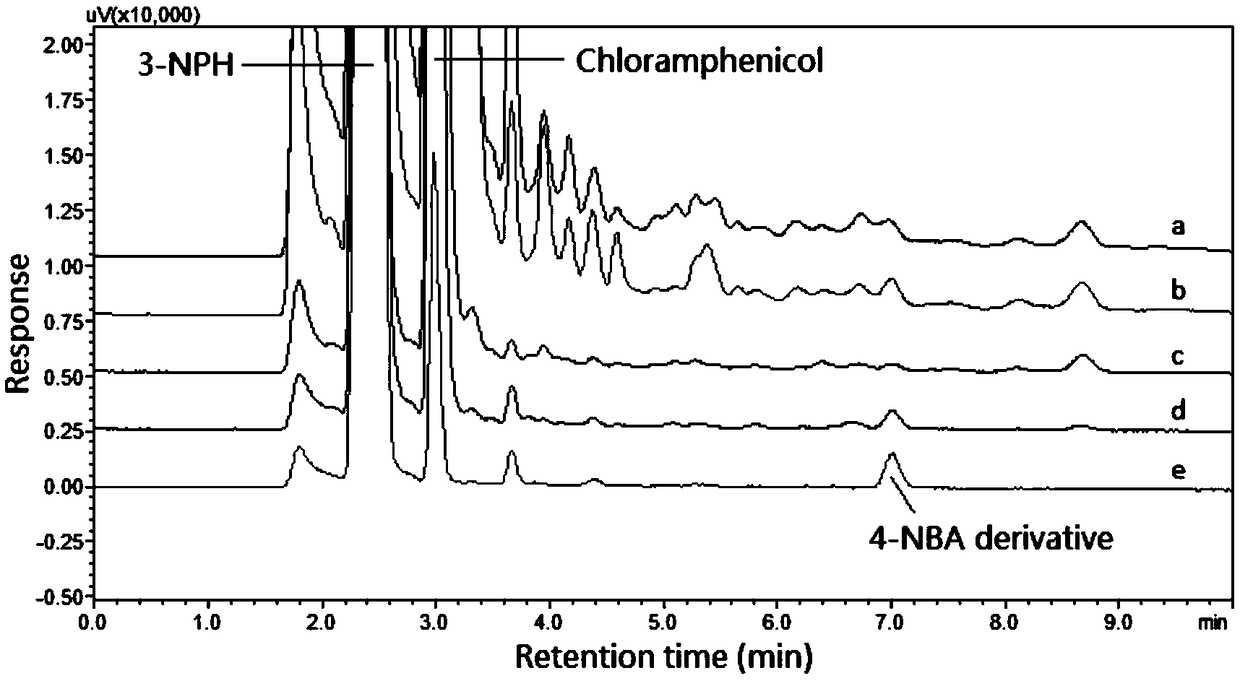
Method for determining 4-nitrobenzaldehyde in chloramphenicol or preparations thereof through derivatization HPLC-UV/Vis
InactiveCN108828089AImprove stabilityWon't interfereComponent separationChemistryQuantitative determination
The invention discloses a method for determining 4-nitrobenzaldehyde in chloramphenicol or preparations thereof through derivatization HPLC-UV / Vis. The method comprises the following steps: subjecting4-nitrobenzaldehyde to derivatization generation of a product with strong absorption in an ultraviolet-visible light zone in virtue of a nitrophenylhydrazine derivatization reagent; and with a reaction solution as a loading sample, determining the derivatization products of 4-nitrobenzaldehyde in the ultraviolet-visible light zone by using HPLC-UV / Vis based on the principle of reversed phase partition chromatography so as to realize qualitative or quantitative determination of 4-nitrobenzaldehyde. According to the invention, since the 4-nitrobenzaldehyde derivatization products based on the nitrophenylhydrazine derivatization reagent have obvious bathochromic shift effect due to generation of hydrazone bonds at nitro para-positions, the simple and universal method for determining 4-nitrobenzaldehyde through pre-column derivatization HPLC-UV / Vis is established. Methodological verification results show that the method has good specificity.
Owner:CHINA PHARM UNIV
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1271040CNo pollution in the processLow costOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
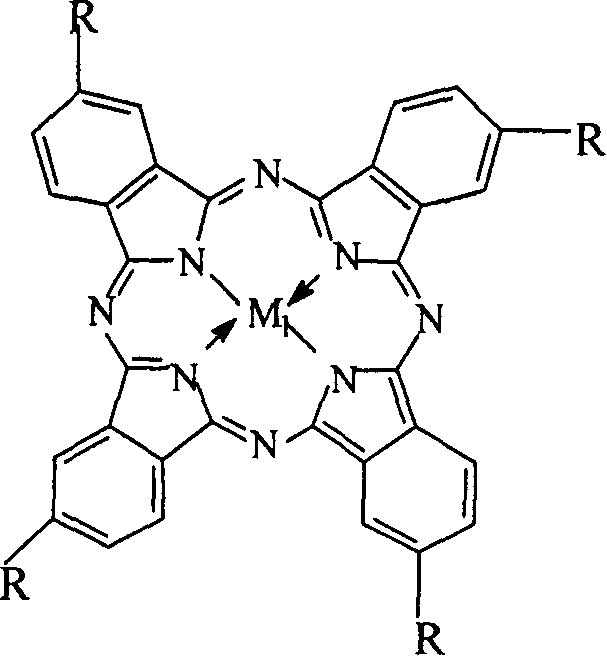
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Mildew resistant medicament and application thereof
InactiveCN1799355ASolve the permanent mildew problemSolve the mildew problemBiocideAnimal repellantsMildewPara-nitrobenzaldehyde
The invention provides a medical agent for preventing the information-recording material from rotting, used for preventing various disk and mag tape from rotting. The long-acting mildew-resistant medicinal agent is comprised with the following raw material with the weight proportion being: m-nitrobenzaldehyde: 10-70%, ª‡-benzalacet aldehyde bromide: 10-70%, 2-hydroxybenzoic acid: 5-40%, geoceric acid: 0.5-20%. The medicine can release the mildew-resistant medical agent of many times as much as that of lowest bacteria inhibitor, possesses high effective killing or inhibiting ability for the common fungus in air, and can preventing the information recording material such as various disk, mag tape and compact disk from rotting.
Owner:华中科技大学同济医学院
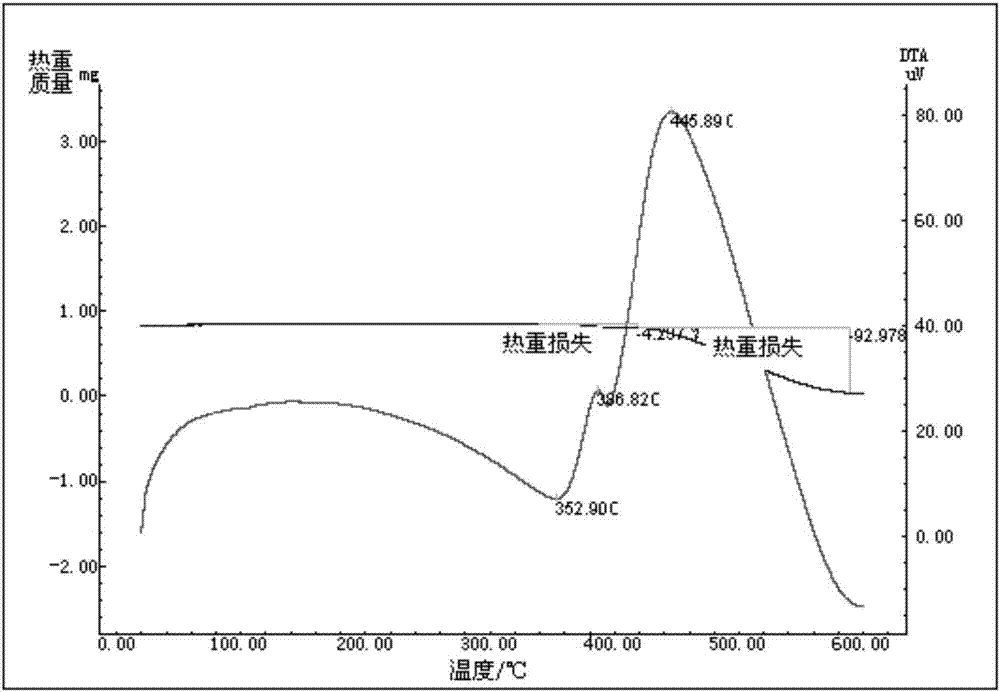
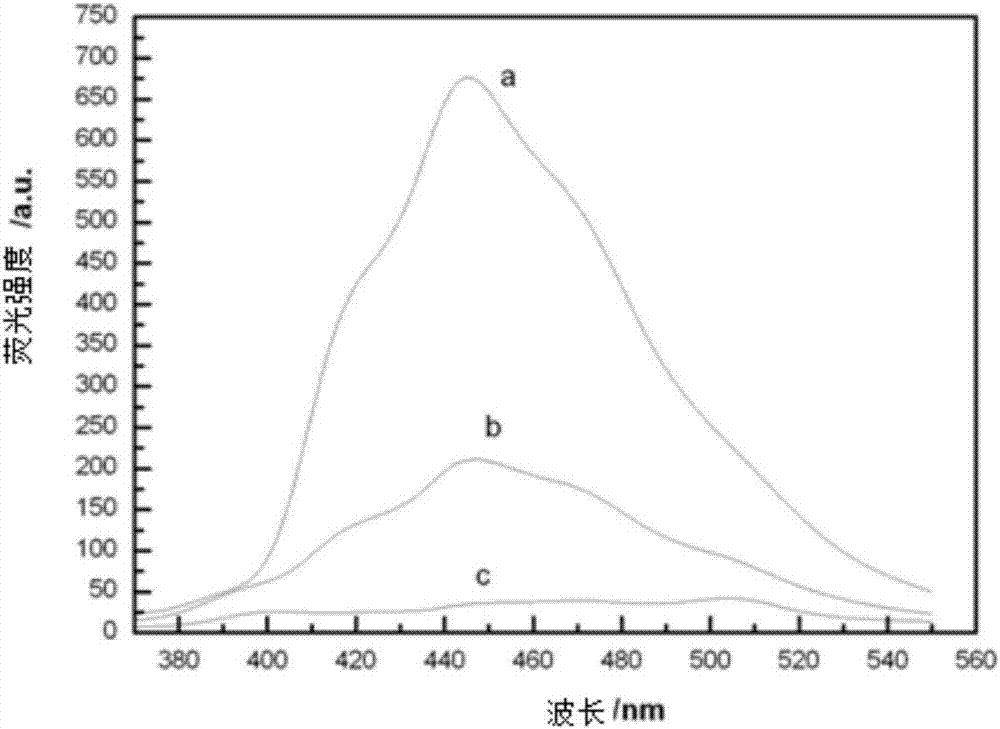
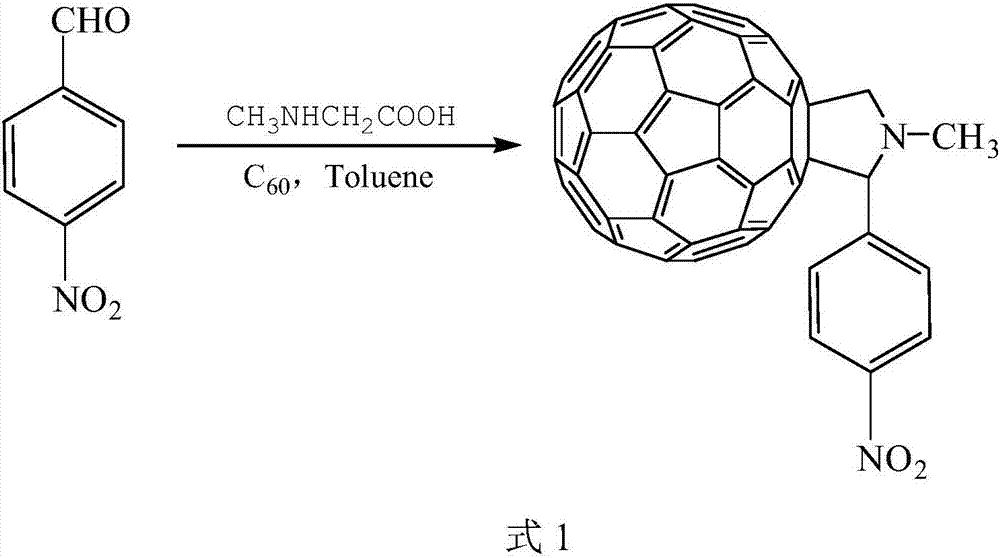
Preparation method of energetic fullerene thin film in petal-shaped microstructure
InactiveCN103497143AOvercome structureOvercome areaOrganic chemistryNanotechnologyOrganic solventNitrogen
The invention relates to a preparation method of an energetic fullerene thin film in a petal-shaped microstructure. The preparation method comprises the steps of (1) in nitrogen protection, implementing reflux reaction to fullerene C60, p-nitrobenzaldehyde and sarcosine in an organic solvent, concentrating, separating and purifying, and recrystallizing to obtain a N-methyl-2-(4-nitrophenyl) [60] fullerene pyrrolidine derivative; (2) dissolving the N-methyl-2-(4-nitrophenyl) [60] fullerene pyrrolidine derivative in an organic solvent, dropping on a substrate, naturally drying and self-assembling to obtain the energetic fullerene thin film in the petal-shaped microstructure. The fullerene derivative obtained from the preparation method is high in fullerene content, rapid and simple in preparation method, and wide in the adopted substrate and contains energetic group nitro; the fullerene thin film material in the petal-shaped microstructure can be prepared in a large area, and has potential application prospect in catalytic materials, photoelectrical materials, energy storage materials and other fields.
Owner:DONGHUA UNIV
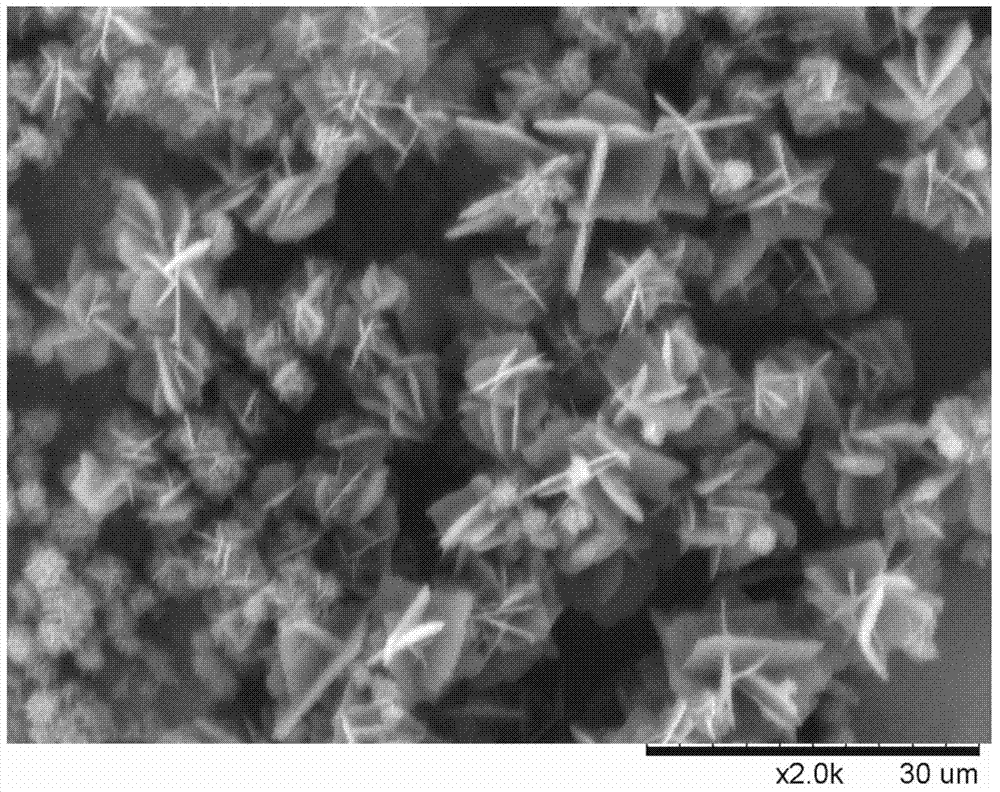
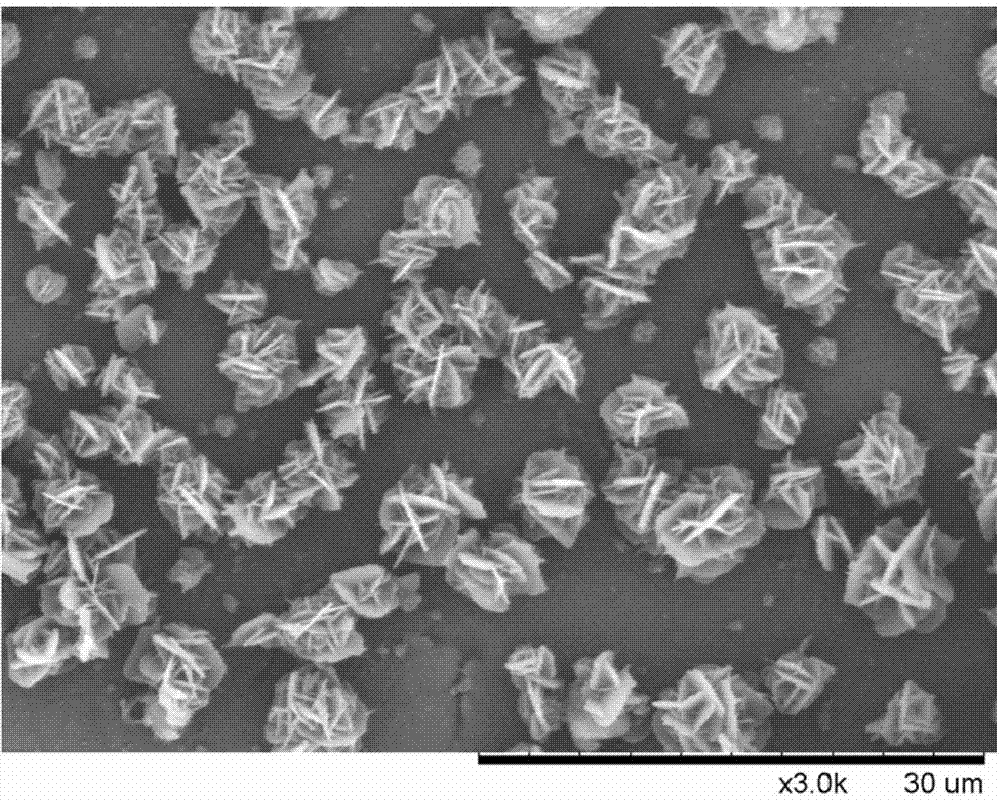
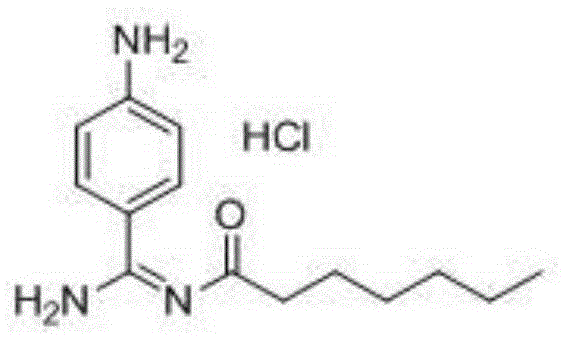
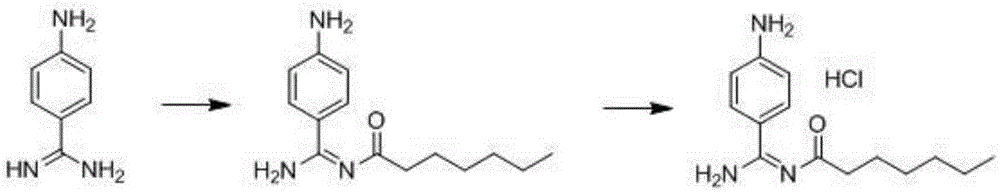
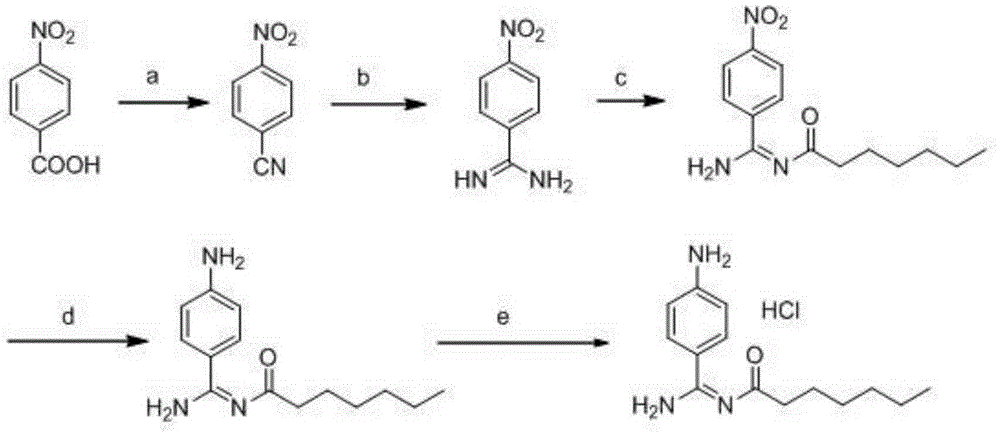
Preparation method for p-aminobenzamidine hydrochloride
InactiveCN105330568AEasy to operateEasy to controlPreparation by nitrogen oxide-organic compound reactionP-aminobenzamidineDrugs synthesis
The invention discloses a preparation method for p-aminobenzamidine hydrochloride, and belongs to the field of drug synthesis. The preparation method comprises the following steps: taking p-aminobenzamidine as a starting raw material, and enabling the p-aminobenzamidine and hydroxylamine hydrochloride to generate nitrobenzonitrile; then, enabling the nitrobenzonitrile to react with ammonium salt to generate p-aminobenzamidine; carrying out acylation and reduction on the p-aminobenzamidine to obtain p-aminobenzamidine imidogen n-hexyl formate; finally, performing salifying on the p-aminobenzamidine imidogen n-hexyl formate and hydrogen chloride to obtain the p-aminobenzamidine hydrochloride. Compared with the prior art, the preparation method for the p-aminobenzamidine hydrochloride disclosed by the invention has the characteristics of being simple to operate, easy to control in reaction, low in production cost and the like, and has a very good popularization and application value.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Corrosion-resistant plastic and preparation method thereof
The invention discloses corrosion-resistant plastic and a preparation method thereof. The corrosion-resistant plastic is prepared from the following raw materials in parts by weight: 80-100 parts of polyvinyl chloride, 5-8 parts of corrosion-resistant filler, 1-3 parts of a dispersing agent, 3-5 parts of a plasticizer and 3-5 parts of a heat stabilizer. The corrosion-resistant filler has a very good corrosion-resistant effect, and the heat stabilizer is prepared through the following steps: taking methyltrimethoxysilane and dimethyldimethoxysilane as raw materials, preparing organic silicon resin through reaction, reducing p-nitrobenzaldehyde by iron powder, reducing nitryl into amino, preparing an intermediate 1, enabling the intermediate 1 to react with phosgene, and preparing an intermediate 2; enabling the intermediate 2 and the 6-amino-1, 3-dimethyl uracil to react to prepare an intermediate 3, and enabling the isocyanate group on the intermediate 3 and the hydroxyl group on the organic silicon resin to react to prepare the thermal stabilizer, such that the thermal stability of the plastic can be improved so as to prolong the service life of the plastic;.
Owner:YUHUAN DEGU NEW MATERIAL TECH CO LTD
Preparation method of N-methyl-2-(4-nitrophenyl)-3,4-fulleropyrrolidine
A preparation method of N-methyl-2-(4-nitrophenyl)-3,4-fulleropyrrolidine comprises the following steps: (1) dissolving C60 powder into a non-polar solvent, thus obtaining a solution; (2) adding sarcosine and p-nitrobenzaldehyde into the solution obtained in the step (1), heating and refluxing; (3) cooling a mixed solution after completing reflux, and removing methylbenzene, thus obtaining a solid product; (4) eluting the solid product obtained in the step (3) by using a chromatographic column; (5) removing a solvent from a product solution obtained through eluting, washing and drying, thus obtaining a target product. The preparation method disclosed by the invention is simple, and the product is high in purity and high in yield.
Owner:HUANGSHAN UNIV
Compounding method for 4,4'-diaminodiphenylmethane
InactiveCN105294446ALow requirements for production equipmentSimple processOrganic compound preparationAmino compound preparationWater bathsNitrobenzene
The invention discloses a compounding method for 4,4'-diaminodiphenylmethane and belongs to the technical field of organic compounding. The compounding method comprises the following steps: firstly, adding formaldehyde solution, hydrogen chloride solution and sulfuric acid solution into nitrobenzene, thereby generating p-nitrobenzaldehyde; adding HNO3 solution and H2SO4 solution into phenol, and then uniformly stirring, and performing water bath temperature rise and ice bath temperature decrease, thereby acquiring p-nitrophenol; mixing p-nitrobenzaldehyde and p-nitrophenol which are acquired at two times, and adding Fe, thereby further generating 4,4'-dinitrobenzene methyl alcohol; lastly, adding zinc powder and hydrogen peroxide solution into acquired 4,4'-dinitrobenzene methyl alcohol, performing centrifugal separation, distilling and drying, thereby acquiring 4,4'-diaminodiphenylmethane. The embodiment proves that the method has a certain universality, the reaction is mild, the technique is simple, the requirement for production equipment is low and the yield of prepared 4,4'-diaminodiphenylmethane reaches more than or equal to 85%.
Owner:高大元
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546458AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationPorphyrinCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Synthetic method of 2-chloroethyl hydrazono methyl formate
InactiveCN109180535AHigh yieldImprove catalytic performanceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAcetic anhydrideDouble phase
The invention discloses a synthetic method of 2-chloroethyl hydrazono methyl formate. According to the synthetic method, dimethyl carbonate, hydrazine hydrate, chloroacetaldehyde, methyl formate, p-nitrobenzaldehyde, acetic anhydride, pyrrole, pyridine, zinc chloride, and terephthalaldehyde are taken as main raw materials; dimethyl carbonate and hydrazine hydrate are subjected to oxidation reaction under the effect of catalyst AZO-CMP-1; chloroacetaldehyde and methyl formate addition substation is carried out so as to obtain the target product 2-chloroethyl hydrazono methyl formate; in oxidation reaction, the catalyst is a liquid solid double-phase catalyst system, and is extremely high in catalytic activity, selectivity, and recycling stability; high conversion degree is achieved; production separation purifying is easy; and product yield is high.
Owner:XUZHOU NORMAL UNIVERSITY
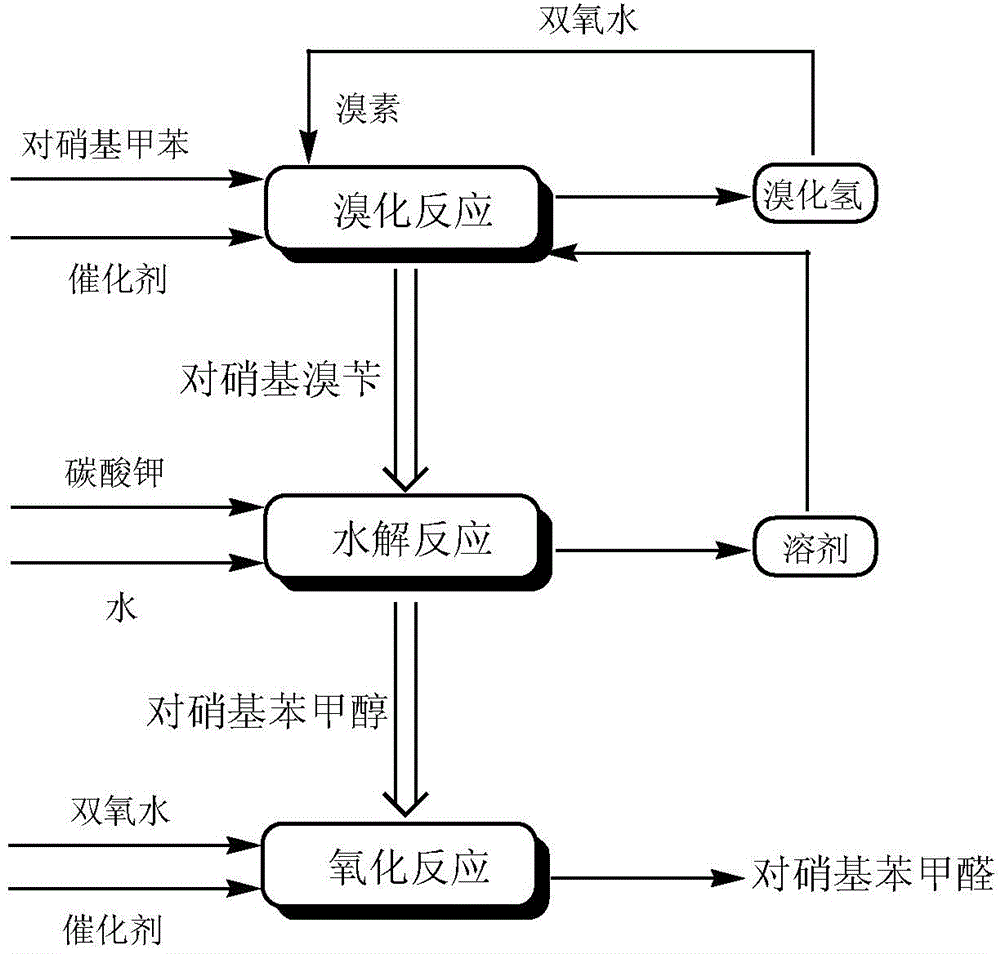
Production process of p-nitrobenzaldehyde
InactiveCN102329235ASolve environmental problemsReduce the impactOrganic chemistryOrganic compound preparationP-nitrotolueneSolvent
The invention relates to a production process of p-nitrobenzaldehyde. The production process is characterized in that p-nitrobenzaldehyde is prepared by the sections of bromination, hydrolysis, oxidation, addition, alkali precipitation, refining and the like based on p-nitrotoluene as a raw material. According to the production process provided by the invention, a solvent used when bromination is carried out is dichlorethane, thereby avoiding the environmental protection problem brought by carbon tetrachloride; an unreacted raw material is used as the solvent when oxidation is carried out; when refining is carried out, 80% ethanol is used as the solvent, thereby reducing the influence of an toxic solvent on a human body; and when bromination is carried out, cheap hydrogen peroxide is added, thereby improving the utilization rate of bromine and avoiding the disadvantages of large amount of hydrogen bromide gases on environment and worker operation. Thus, the production process provided by the invention has higher environmental friendliness and safety, and is suitable for industrial production on large scale; and by test, the yield of p-nitrobenzaldehyde is above 65%, and the chromatographic purity of the finished product can reach above 99.5%.
Owner:GUANNAN YISITE CHEM
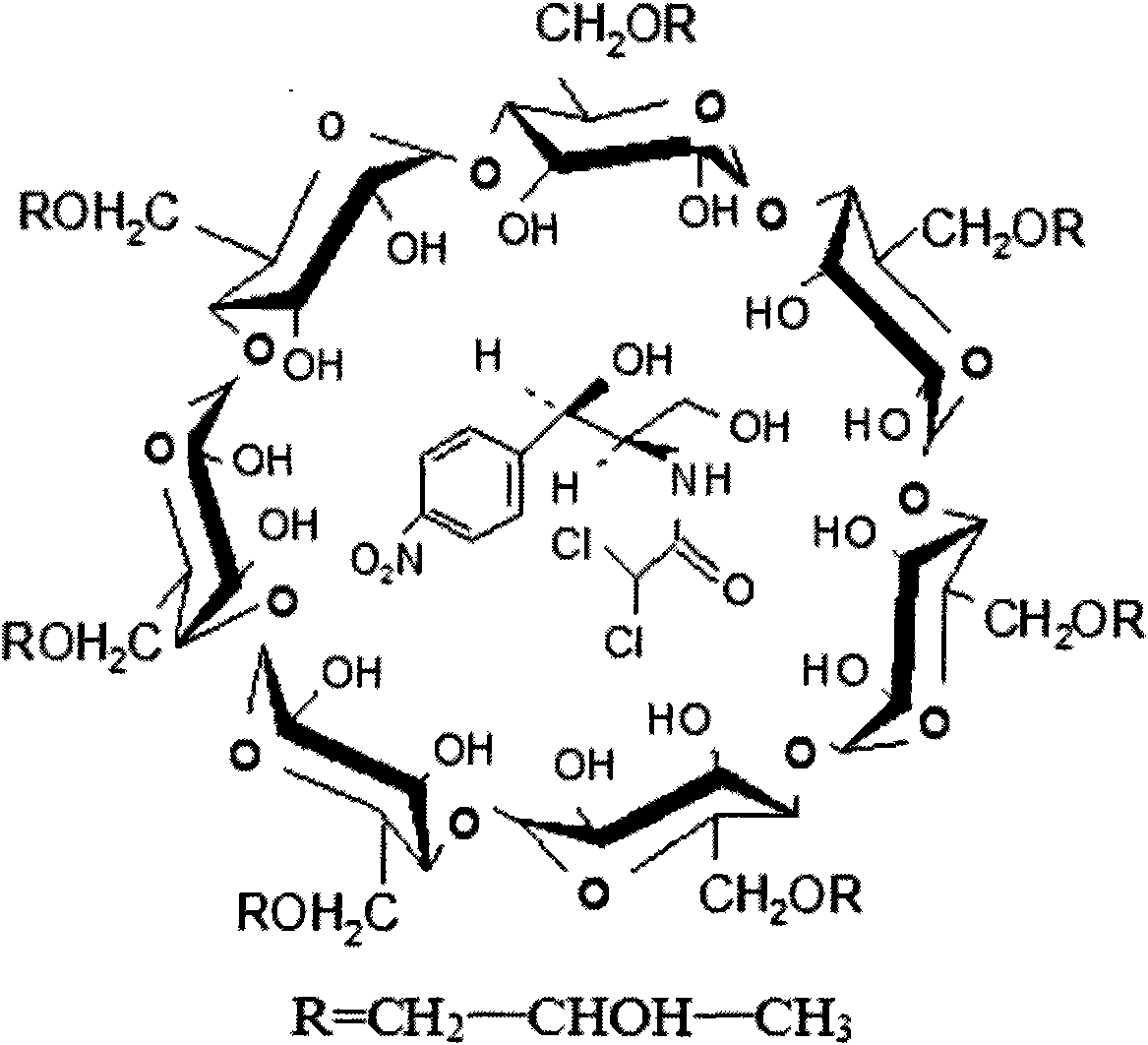
Application of the sulfur-butyl ether-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof
InactiveCN101549160AFast dissolutionBlock hydrolysis reactionOrganic active ingredientsSenses disorderMethyl aldehydeSulfur
The present invention is application of the sulfur-butyl ether-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof. Adding sulfur-butyl ether -belta-cyclodextrin into formula of the eyedrops of chloramphenicol, the addition is 10-80 g / L. The invention finds SBE-belta-CD (sulfur-butyl ether -belta-cyclodextrin) for improving stability of the eyedrops of chloramphenicol by a series of experiments, capable of improving dissolvability of the chloramphenicol for six times, and because of inclusion of the cavity structure to chloramphenicol, blocking chloramphenicol hydrolysis reaction catalyzed by various ions in solution effectively, degradation of the chloramphenicol in aqueous solution is greatly relieved, by experiments, accelerating for six months and normal temperature storing for 24 months, every index of the chloramphenicol eyedrops still conforms to request of the standard, content of chloramphenicol glycol is less than 8%, content of methyl aldehyde is 0, so stability of the chloramphenicol eyedrops is greatly improved.
Owner:昆明振华制药厂有限公司
Method for preparing chloramphenicol
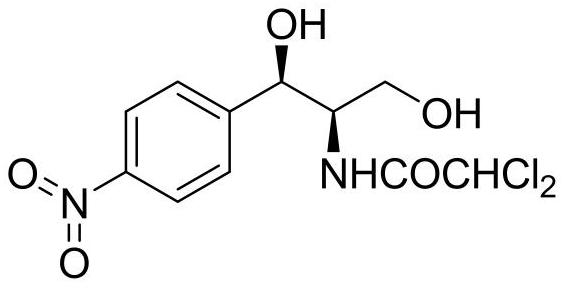
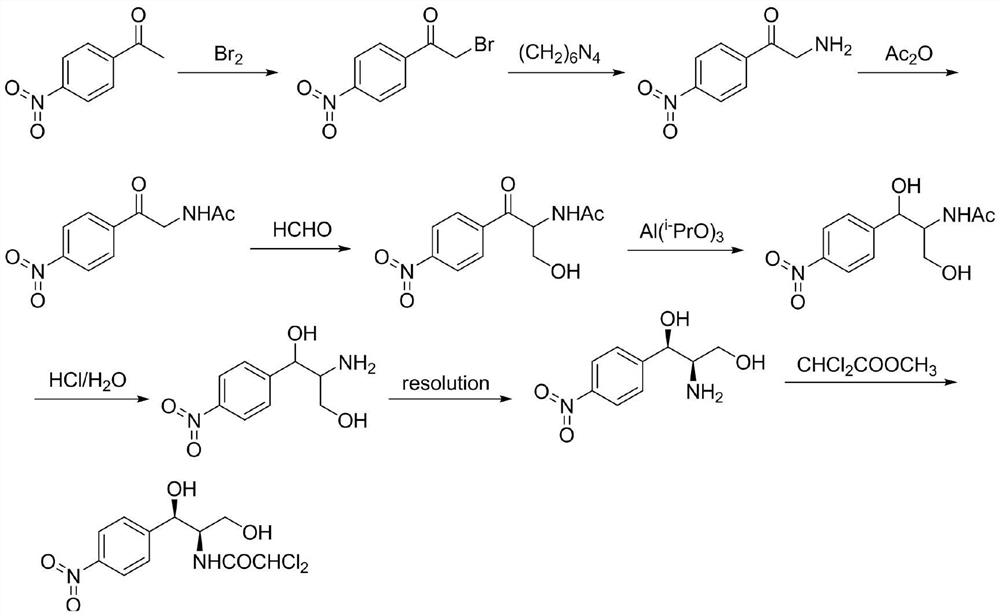
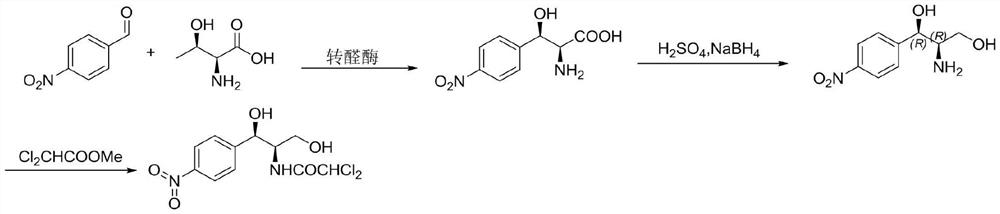
The invention provides a method for preparing chloramphenicol. (2S,3R)-p-nitrophenyl serine is prepared from p-nitrobenzaldehyde and L-threonine which are low in price and are easy to obtain under action of transaldolase, and a target product chloramphenicol is obtained through reduction and dichloroacetylation of the amino acid intermediate.
Owner:湖南引航生物科技有限公司
High selectivity synthesis method of p-nitrobenzaldehyde
InactiveCN102126960BReduce pollutionReduce consumptionOrganic chemistryOrganic compound preparationSynthesis methodsP-nitrotoluene
A high selectivity synthesis method of p-nitrobenzaldehyde comprises the following steps: firstly, adding p-nitrotoluene, peroxycarbonate used as catalyst and dichloroethane used as solvent in a reactor, dropping bromine at 40-50 DEG C under stirring, then reacting at 50-60 DEG C to ensure that the color of bromine fades, adding hydrogen peroxide to react at 60-70 DEG C for no less than 4 hours and prepare 4-nitrobenzyl bormide; and secondly, adding 25-35% sodium carbonate solution to hydrolyze at 80-95 DEG C and generate p-nitrobenzyl alcohol, standing to separate, and finally using oxygen as oxidant to react for no less than 25 hours in the presence of catalyst triphenylphosphine metal salt organic complex under the conditions that the temperature is 50-90 DEG C and the pressure 5.1*10<5>-1.0*10<6>. The overall yield of the method is no less than 70%, the product purity is no less than 99% and the dosage of bromine is 50-60% of the theoretical amount.
Owner:HEFEI UNIV OF TECH
Acidamide derivative preparation method
InactiveCN1544416AAvoid corrosionEasy to operateOrganic compound preparationCarboxylic acid amides preparationStrong acids3-Bromobenzaldehyde
A process for preparing amide derivant, comprises reacting fatty group amide with aldehydes in methyl alcohol or carbon tetrachloride or benzene or methylbenzene or dioxane or acetonitrile with the presence of catalyst, and separating the reaction product, characterized in that the catalyst is type H strong acidic positive ion exchange resin, the aldehyde is selected from the groups of paraformaldehyde, acetaldehyde, propionaldehyde, chloral, bromal, glyoxylic acid, 4-bromobenzaldehyde, 3-bromobenzaldehyde, hydrosulfide acetaldehyde, p-nitrobenzaldehyde, and 4-hydroxybenzaldehyde. The formula of the fatty group amide is described in the specification, wherein R 2 is saturated or unsaturated fatty group having 2-12 carbon atoms, the type H strong acidic positive ion exchange resin is sulphonated coal, sulfonated phenylethene copolymer or sulfonated phenol formaldehyde resin.
Owner:OCEAN UNIV OF CHINA
Specific porphyrin self-transport nano carrier material and preparation method thereof
ActiveCN112121166ALow costSave raw materialsOrganic active ingredientsEnergy modified materialsPropanoic acidAcetic anhydride
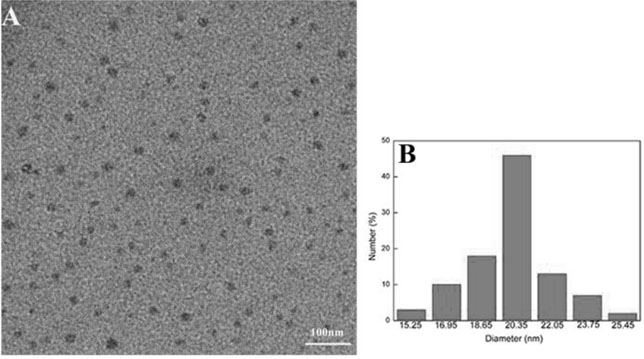
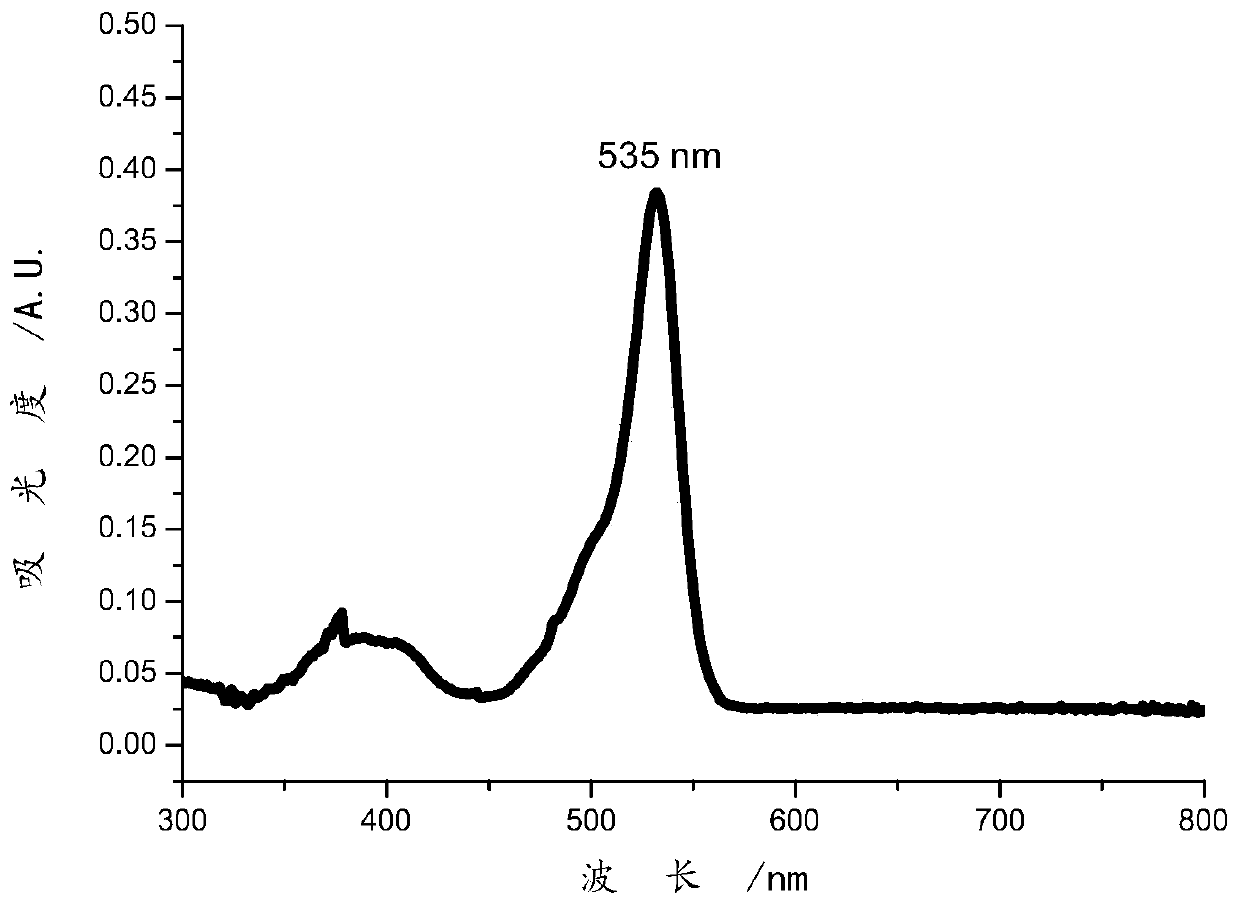
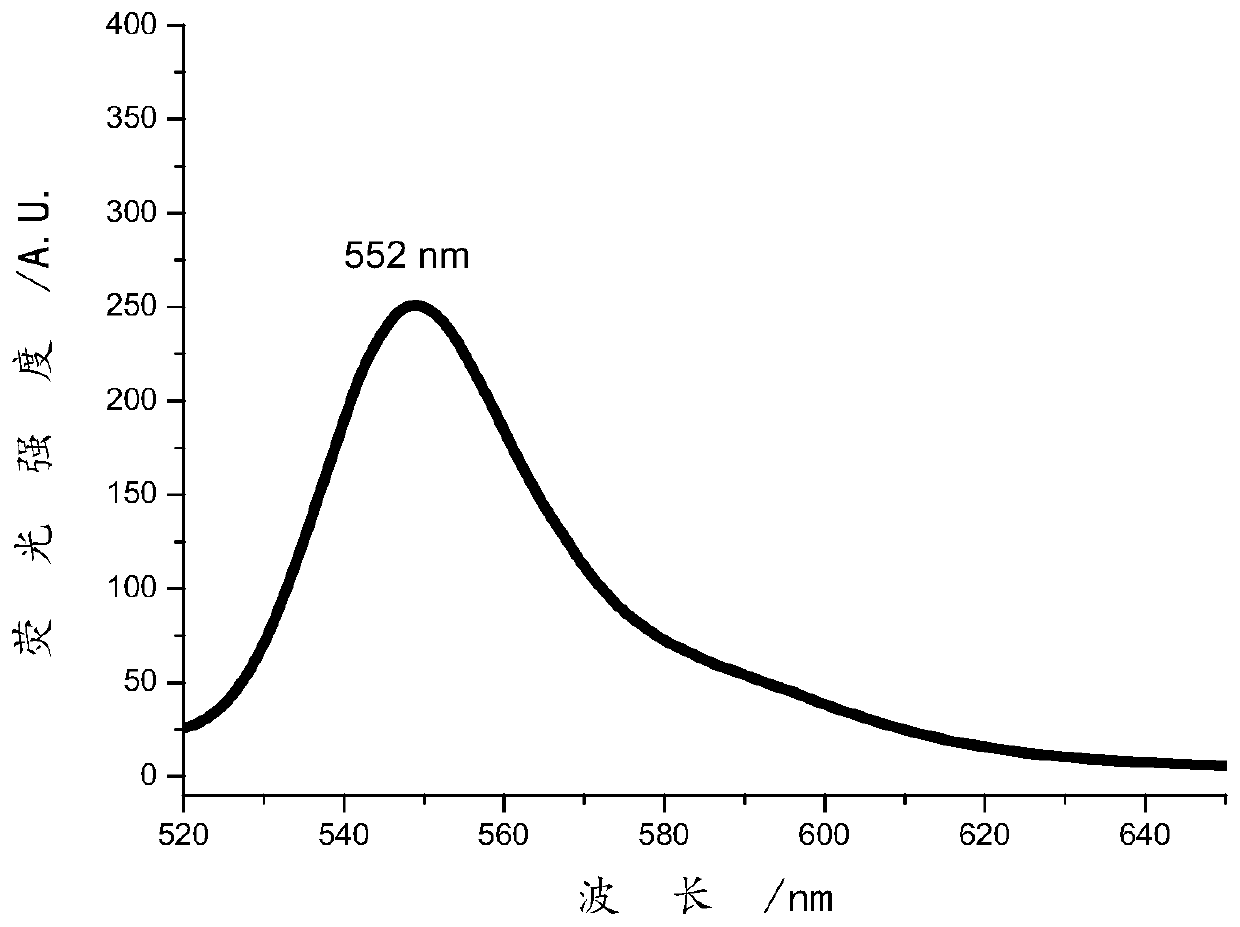
The invention relates to a preparation method of a specific porphyrin self-transport nano carrier material. The method comprises the following steps of carrying out a mixed reaction on p-nitrobenzaldehyde, acetic anhydride, propionic acid and pyrrole to obtain purple solid tetra(4-nitrophenyl)porphyrin TNPP, and preparing tetra(4-aminophenyl)porphyrin TAPP; stirring TPGS, succinic anhydride, DMAP,triethylamine and 1,4-dioxane at room temperature, and performing dialysis to obtain carboxyl TPGS; and mixing the carboxyl TPGS, DMAP and PyBOP, adding DMF and TAPP, performing stirring at room temperature for 22-26 hours, performing dialysis, and freeze-drying dialysate to obtain the product TAPP-TPGS. The specific molecules TPGS are introduced into the carrier material, so that the carrier hasthe effect of assisting an anti-tumor drug in treating cancers, and the anti-tumor effect is improved through combined treatment of three modes of TPGS, PDT and chemotherapy.
Owner:HENAN UNIVERSITY
Halogen-free low-smoke flame-retardant wire and cable and preparation method thereof
PendingCN113652044ALarge heat capacityChemically activePlastic/resin/waxes insulatorsCopper conductorPtru catalyst
The invention relates to a halogen-free low-smoke flame-retardant electric wire and cable and a preparation method thereof, the halogen-free low-smoke flame-retardant electric wire and cable comprises a copper conductor and an insulating layer coating the copper conductor, and the insulating layer comprises the following components in parts by weight: 100-120 parts of polytetrafluoroethylene; 30 to 40 parts of 2-(4-methoxyphenyl) malonaldehyde; 5-6 parts of silicon resin; 12 to 15 parts of aluminum hydroxide; 8-10 parts of ricinoleic acid; 5 to 6 parts of p-nitrobenzaldehyde; and 1-2 parts of a catalyst. The preparation method of the halogen-free low-smoke flame-retardant electric wire and cable comprises preparation of an electric wire and cable material of an insulating layer and a cabling process. The halogen-free low-smoke flame-retardant electric wire and cable has the following advantages and effects: 2-(4-methoxyphenyl) malonaldehyde is easy to combine with polytetrafluoroethylene, and has a good flame retardant effect; the aluminum hydroxide reduces conduction heat resistance and is not easy to ignite; and in the presence of a catalyst, the aluminum hydroxide is modified by a product obtained by mixing the ricinoleic acid and the p-nitrobenzaldehyde, so that a good bonding interface is formed to form a continuous closed structure, and the flame retardant property is improved.
Owner:温州市电线二厂
Synthesis method of 2-methyl quinoline
Owner:XUZHOU NORMAL UNIVERSITY
Photosensitizer probe as well as preparation method and application thereof
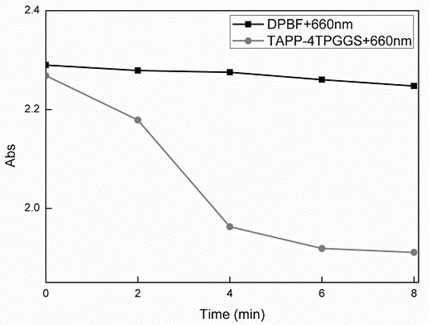
ActiveCN111592560AEfficient singlet oxygen generation capabilityThe preparation method is simple and safePhotodynamic therapyGroup 3/13 element organic compoundsPalladium on carbonSinglet oxygen
The invention discloses a photosensitizer probe as well as a preparation method and application thereof. The preparation method comprises the following steps: in a nitrogen atmosphere, dissolving p-nitrobenzaldehyde, 2,4-dimethylpyrrole and trifluoroacetic acid into a solvent, reacting under stirring, adding 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, quickly adding triethylamine and a boron trifluoride diethyl ether complex under an ice bath condition, stirring at room temperature, and treating a reaction product to obtain an intermediate 1; dissolving the intermediate 1 and N-iodosuccinimideinto dichloromethane, stirring at room temperature, and treating a reaction product to obtain an intermediate 2; adding palladium on carbon, the intermediate 2, hydrazine hydrate and ethanol, performing a reflux stirring reaction, cooling to room temperature, and treating a reaction product to obtain an intermediate 3; dissolving the intermediate 3 and an alkali in a solvent, dropwise adding an acetyl chloride solvent, reacting under stirring and treating a reaction product to obtain the photosensitizer probe ACDB. The photosensitizer probe shows efficient singlet oxygen generation capacity and fluorescence emission capacity, and has the potential of being used for real-time monitoring of tumor photodynamic therapy and fluorescence imaging.
Owner:NANJING UNIV OF TECH
Synthetic preparation method of stable isotope labeled phenylethanolamine A
InactiveCN111777518AReduce manufacturing costReduce experimental stepsOrganic compound preparationOrganic chemistry methodsNitrobenzeneAcetophenone
The invention discloses a synthetic preparation method of stable isotope labeled phenylethanolamine A. The synthetic preparation method comprises the following steps of: S1) firstly reacting p-nitrobenzaldehyde with acetyltriphenylphosphine, and then conducting reducing by trichlorosilane to obtain a compound shown as formula 1; S2) carrying out reductive amination reaction on the compound shown as formula 1 to obtain a compound shown as formula 2; S3) subjecting bromo-p-hydroxyacetophenone and deuterated methanol to Mmitsunobu reaction so as to obtain deuterium-labeled bromo-p-methoxyacetophenone shown as formula 3; and S4) carrying out nucleophilic reaction on the compounds shown as formula 2 and formula 3 in methanol, and directly performing reduction by using a hydroboration reagent toobtain the target compound stable isotope labeled phenylethanolamine A. According to the method, cheap p-nitrobenzaldehyde and p-hydroxyacetophenone are used as the raw materials, phenylethanolamineA with purity and abundance both up to 98% or above can be prepared through simple synthesis of four steps, the experiment steps are few, the operation is simple, and the detection cost is greatly reduced.
Owner:SHANGHAI ANPEL SCI INSTR
Application of the hydroxypropyl-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof
ActiveCN101549159AImprove stabilityGood water solubilityOrganic active ingredientsSenses disorderMethyl aldehydeCyclodextrin
The present invention is application of the hydroxypropyl-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof. Adding hydroxypropyl-belta-cyclodextrin into formula of the eyedrops of chloramphenicol, the addition is 10-80 g / L. The invention finds HP-belta-CD (hydroxypropyl-belta-cyclodextrin) for improving stability of the eyedrops of chloramphenicol by a series of experiments, capable of improving dissolvability of the chloramphenicol for six times, and because of inclusion of the cavity structure to chloramphenicol, blocking chloramphenicol hydrolysis reaction catalyzed by various ions in solution effectively, degradation of the chloramphenicol in aqueous solution is greatly relieved, by experiments, accelerating for six months and normal temperature storing for 24 months, every index of the chloramphenicol eyedrops still conforms to request of the standard, content of chloramphenicol glycol is less than 8%, content of methyl aldehyde is 0, so stability of the chloramphenicol eyedrops is greatly improved.
Owner:YUNNAN PHYTOPHARML
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com