Patents
Literature
76 results about "P-nitrotoluene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Meta-nitrotoluene (MNT), m-nitrotoluene, or 3-nitrotoluene. It is a yellowish-greenish to yellow liquid with weak fragrance. para-nitrotoluene (PNT), p-nitrotoluene, or 4-nitrotoluene. It is a pale yellow material forming rhombic crystals and has a somewhat pleasant, characteristic smell of bitter almonds.
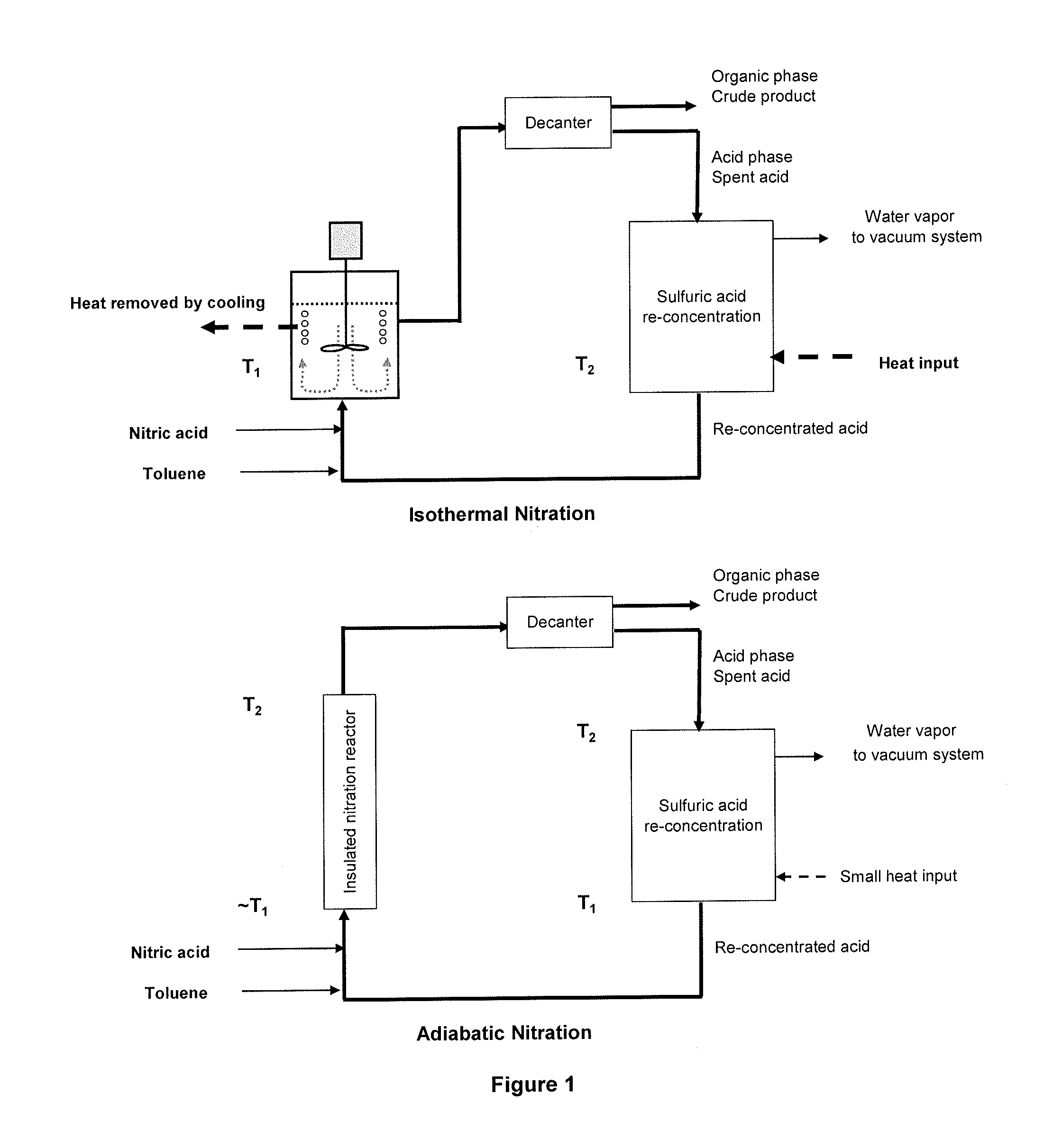
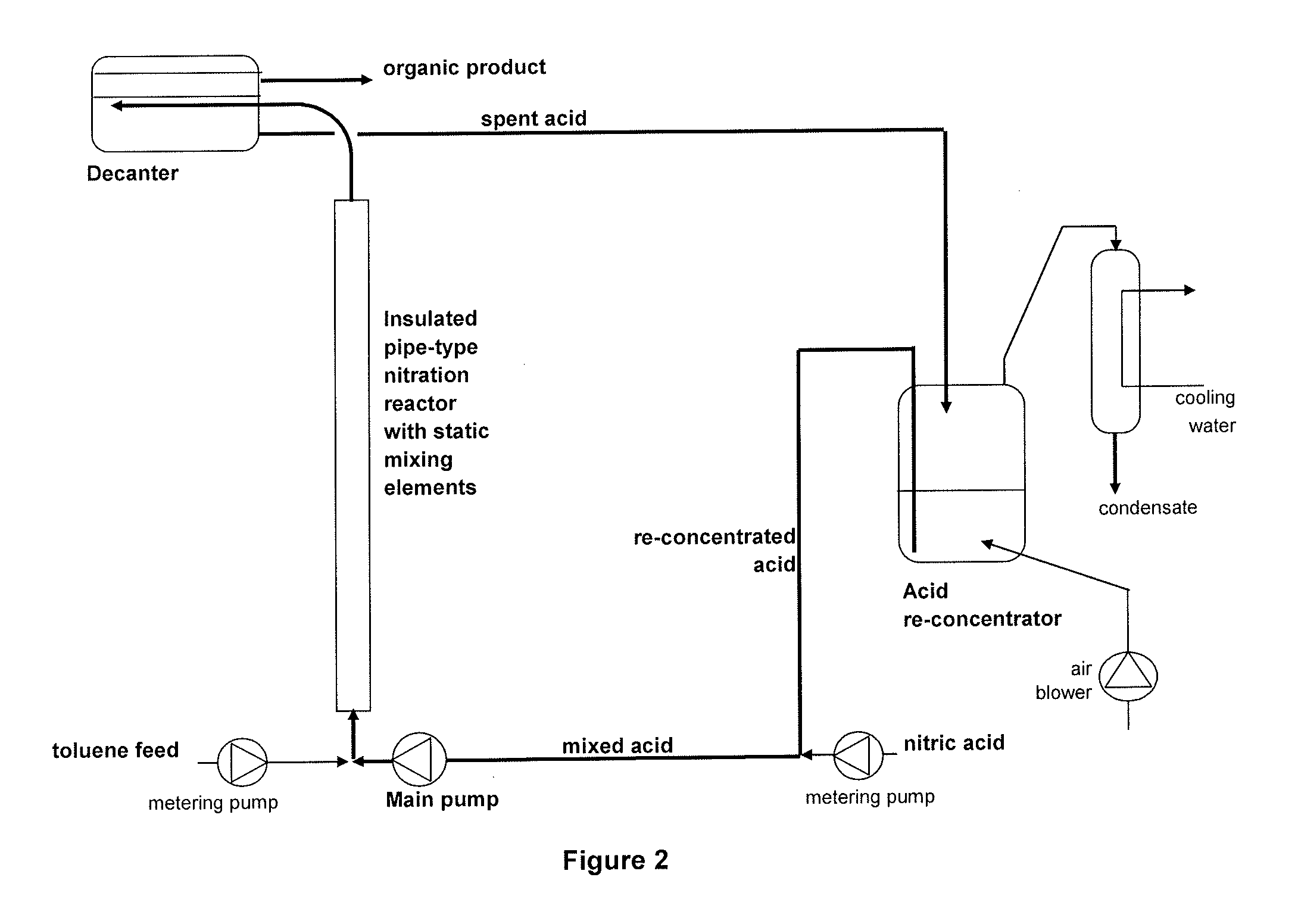
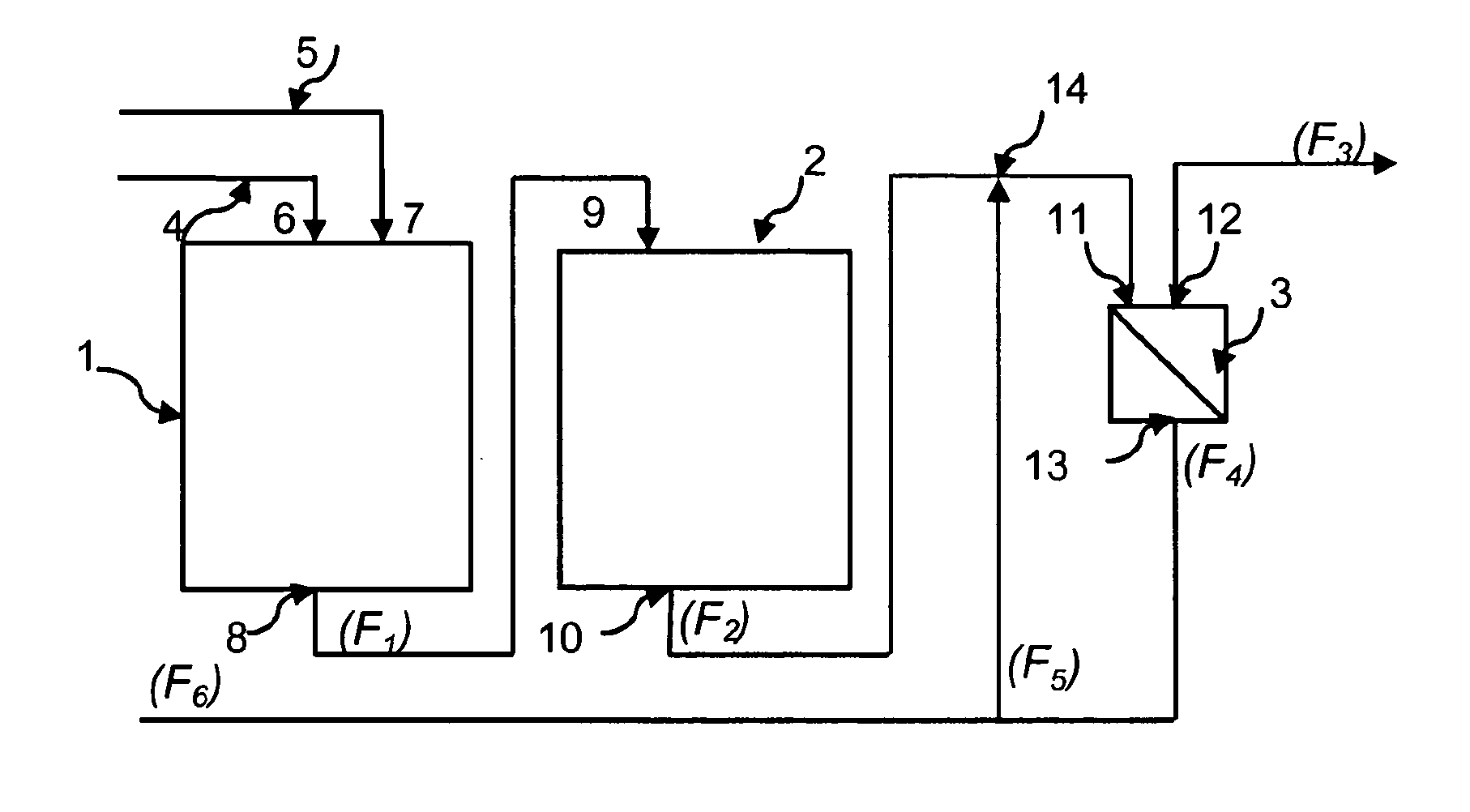
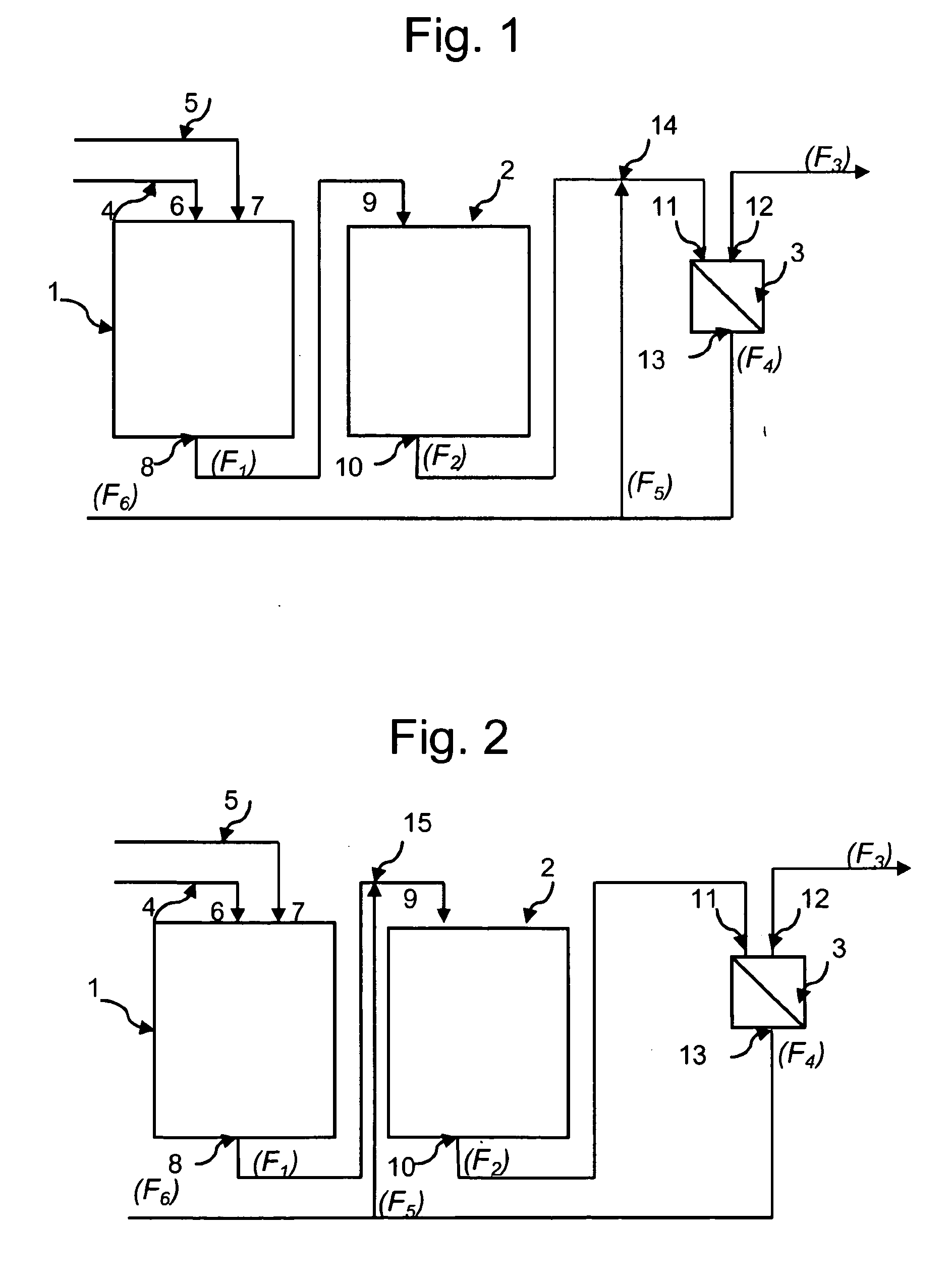
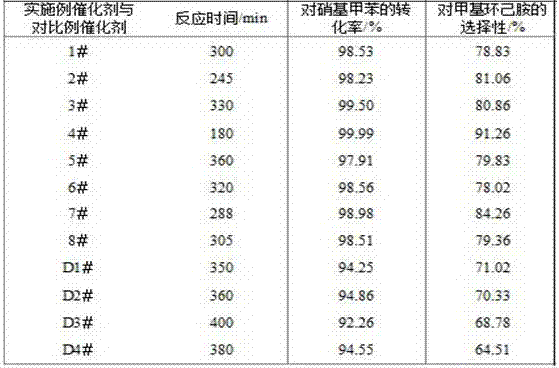
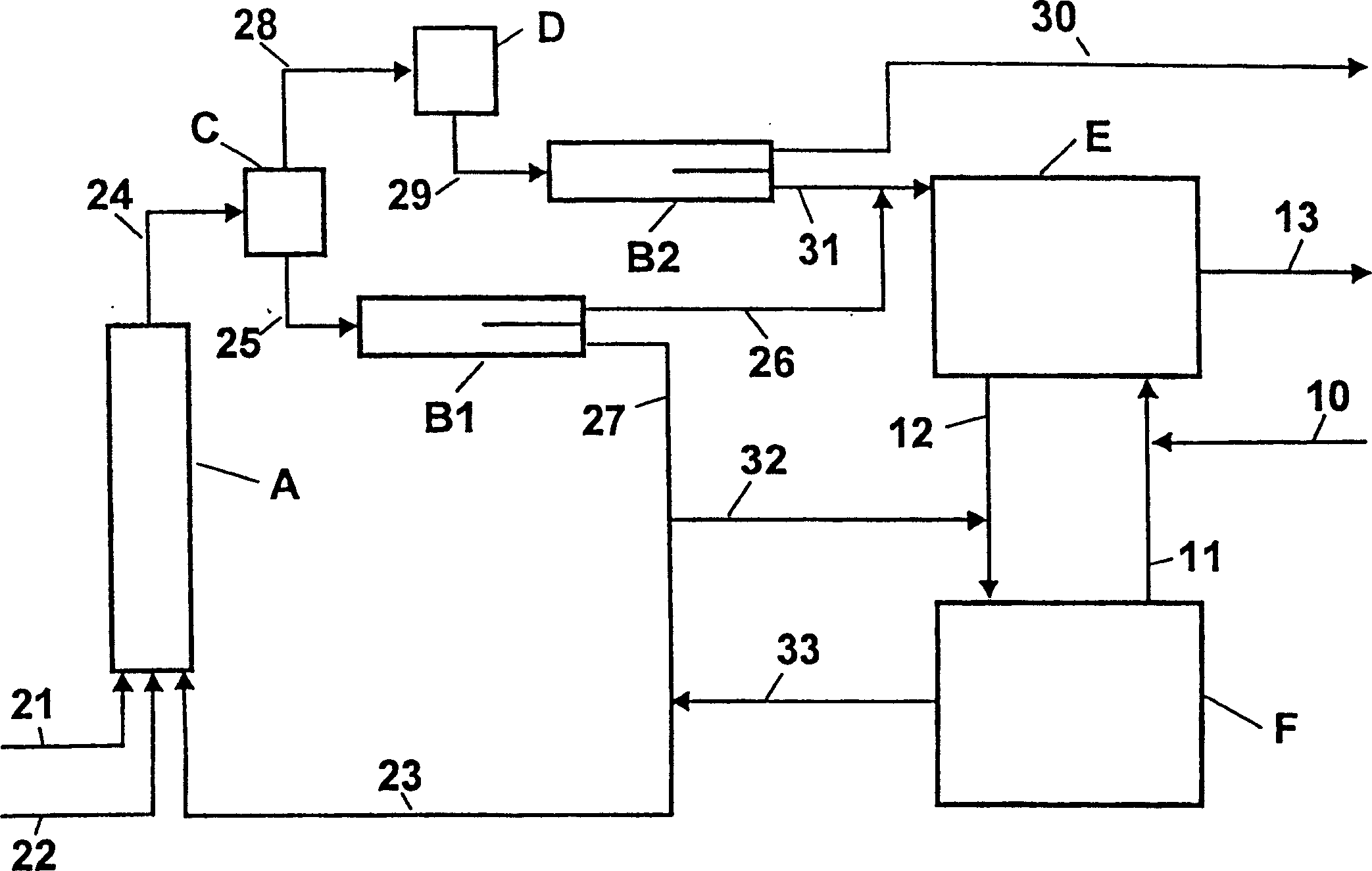
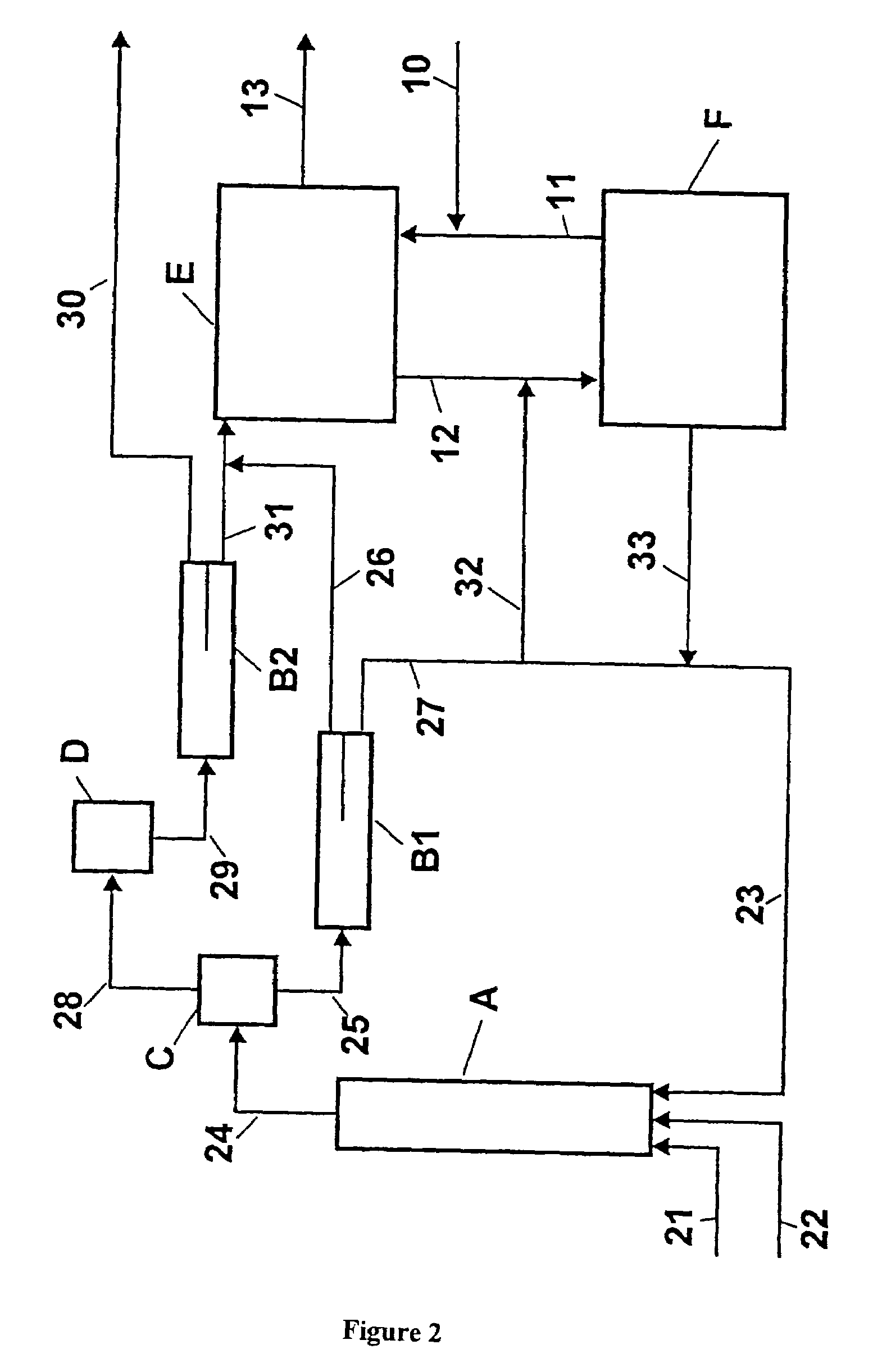
Process for adiabatic production of mononitrotoluene
ActiveUS8907144B2Organic compound preparationOxygen compounds preparation by hydrocarbon oxidationReaction rateNitration
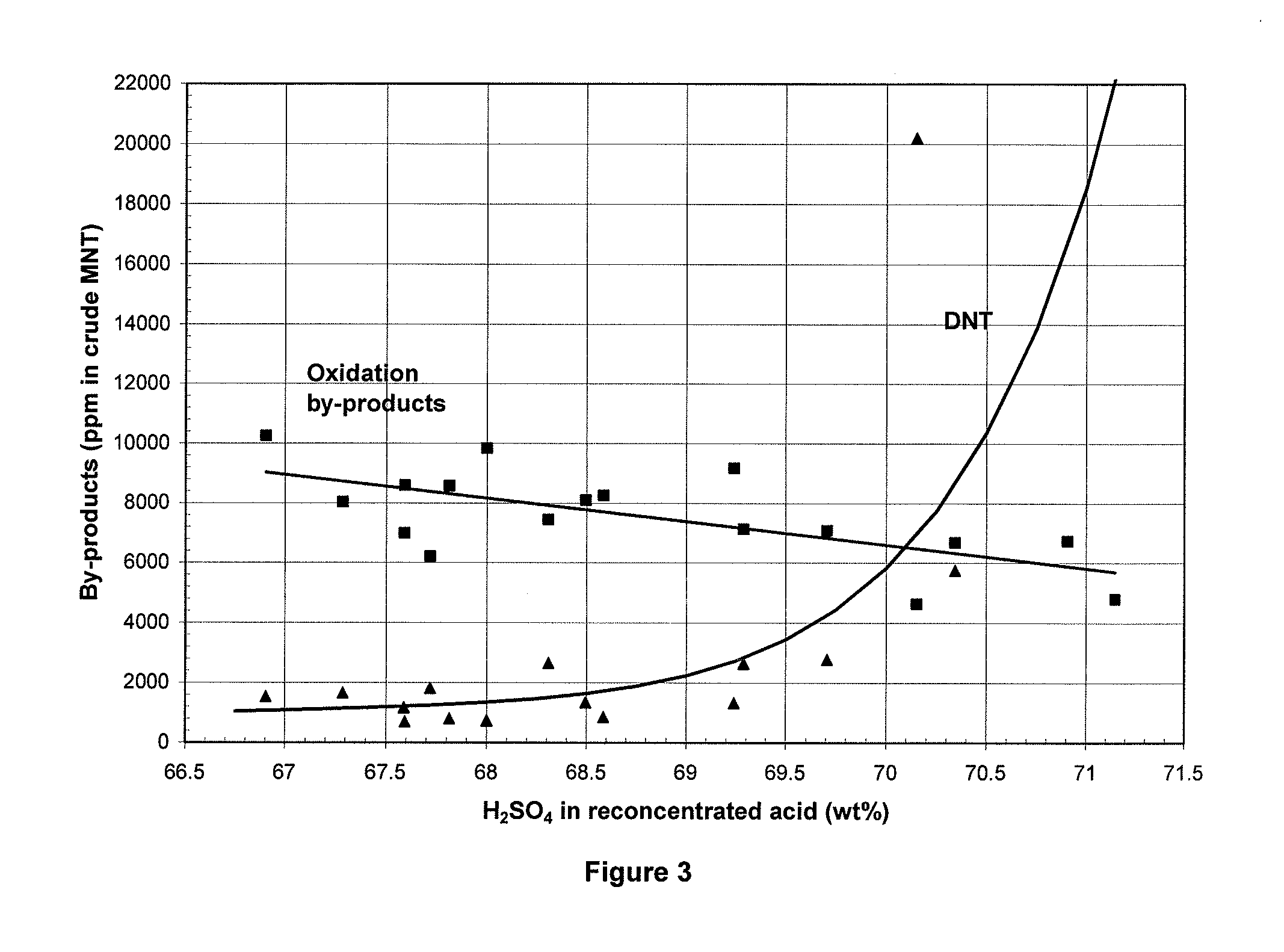
A process for continuous adiabatic nitration of toluene to mononitrotoluene (MNT). The process yields a product quality of MNT that is comparable to that obtained by isothermal production. The process uses excess toluene, with the reaction rate being controlled to maintain a residual of 0.003-0.102 wt % nitric acid in the spent acid and an orange to red color of the spent acid. Further process conditions include re-concentrated sulfuric acid at 83 to 99 degrees C. with a concentration of sulfuric acid from 66 to 70.5 wt %. This is mixed with nitric acid to generate a mixed acid with 1.0 to 3.8 wt % nitric acid and toluene is added at a rate of 1.1 to 1.71 moles toluene / mole nitric acid. The reactants are mixed in a reactor with an overall average mixing intensity of 5.8 to 19 W / kg of contained solution.
Owner:NORAM INT
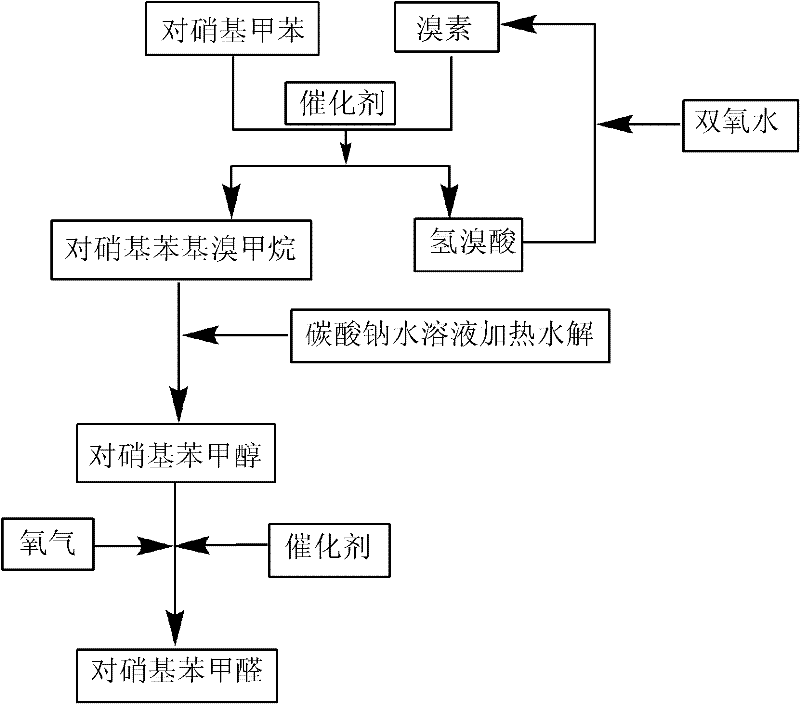
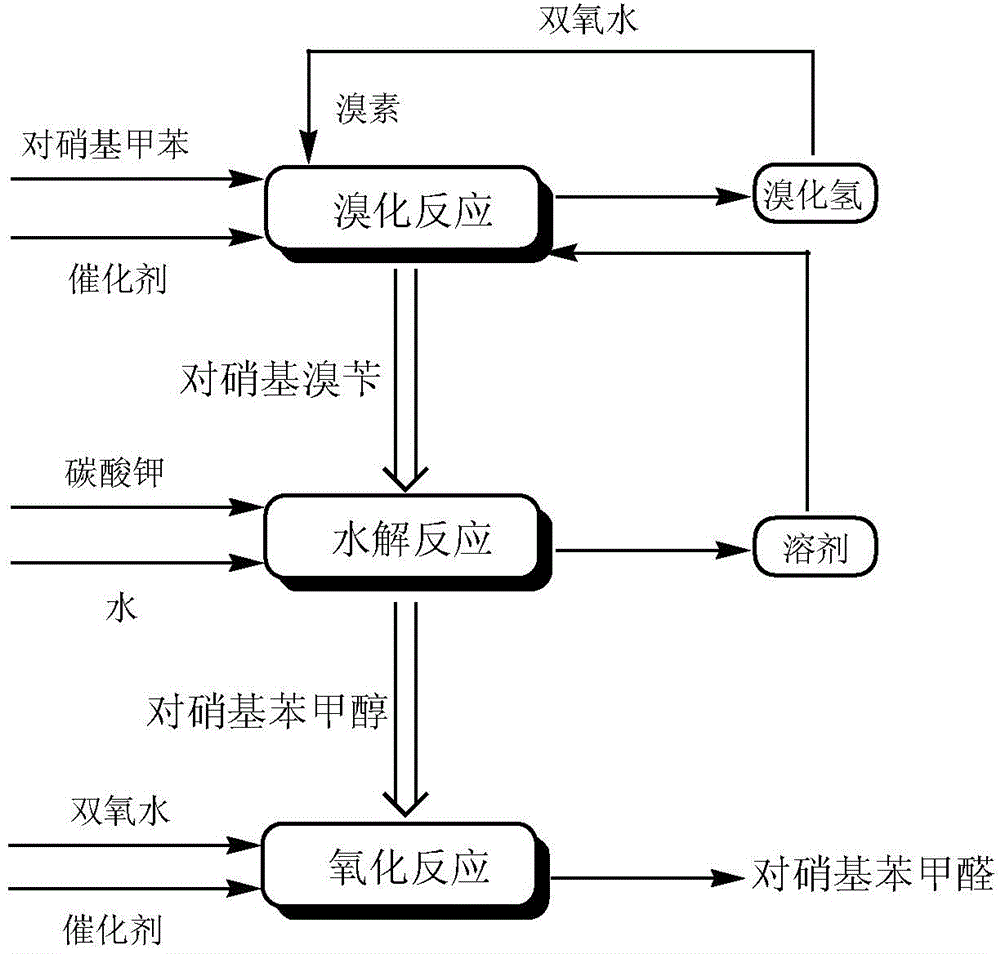
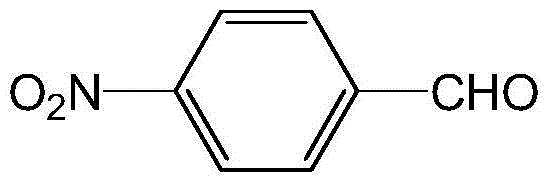
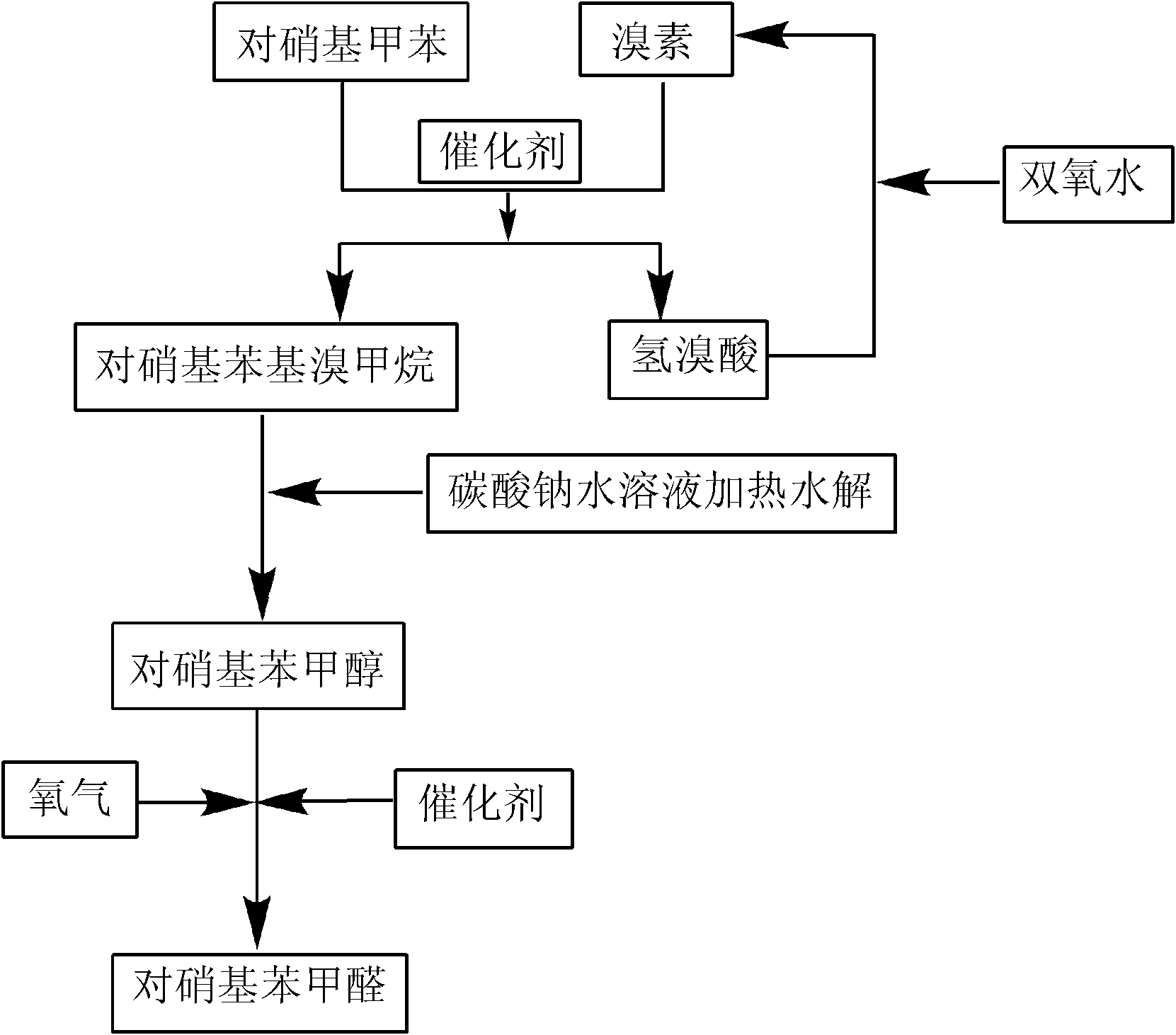
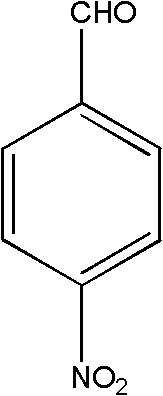
High selectivity synthesis method of p-nitrobenzaldehyde
InactiveCN102126960AReduce pollutionReduce consumptionOrganic chemistryOrganic compound preparationP-nitrotolueneBromine
A high selectivity synthesis method of p-nitrobenzaldehyde comprises the following steps: firstly, adding p-nitrotoluene, peroxycarbonate used as catalyst and dichloroethane used as solvent in a reactor, dropping bromine at 40-50 DEG C under stirring, then reacting at 50-60 DEG C to ensure that the color of bromine fades, adding hydrogen peroxide to react at 60-70 DEG C for no less than 4 hours and prepare 4-nitrobenzyl bormide; and secondly, adding 25-35% sodium carbonate solution to hydrolyze at 80-95 DEG C and generate p-nitrobenzyl alcohol, standing to separate, and finally using oxygen as oxidant to react for no less than 25 hours in the presence of catalyst triphenylphosphine metal salt organic complex under the conditions that the temperature is 50-90 DEG C and the pressure 5.1*10<5>-1.0*10<6>. The overall yield of the method is no less than 70%, the product purity is no less than 99% and the dosage of bromine is 50-60% of the theoretical amount.
Owner:HEFEI UNIV OF TECH
Cost-saving preparation method of p-nitrobenzyl alcohol
InactiveCN104370746ANothing producedNo production, in line with the concept of green productionOrganic chemistryOrganic compound preparationState of artP-nitrobenzyl chloride
The invention discloses a cost-saving preparation method of p-nitrobenzyl alcohol. The key points of the technical scheme are as follows: according to the cost-saving preparation method of the p-nitrobenzyl alcohol, p-nitrotoluene is taken as a starting raw material and reacts with chlorine gas to obtain p-nitrobenzyl chloride, and then the p-nitrobenzyl chloride is hydrolyzed in an alkaline solution to obtain p-nitrobenzyl alcohol. Compared with the prior art, the cost-saving preparation method of the p-nitrobenzyl alcohol has the following beneficial effects: the reaction system is simple and the raw material is cheap and easily available; the preparation cost is low and no hazardous waste is generated, so that the preparation method accords with the green production ideal; the cost-saving preparation method is simple in process, simple and convenient to operate, short in reaction time, high in efficiency and suitable for large-scale production.
Owner:HENAN NORMAL UNIV
Method for synthesizing p-nitrobenzoic acid
InactiveCN109232260ANo cloggingNo pollution in the processOrganic chemistryOrganic compound preparationP-nitrobenzoic acidP-nitrotoluene
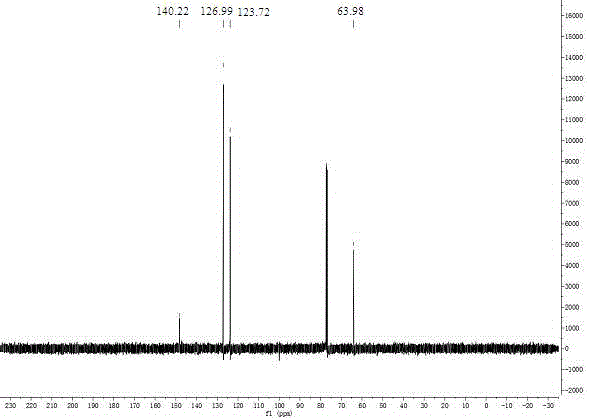
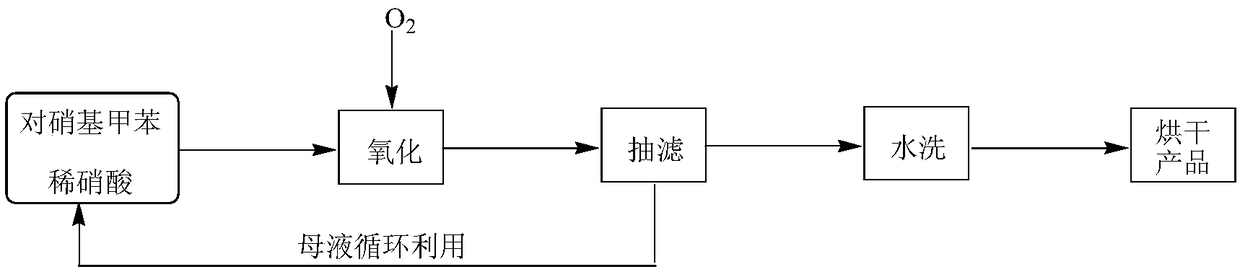
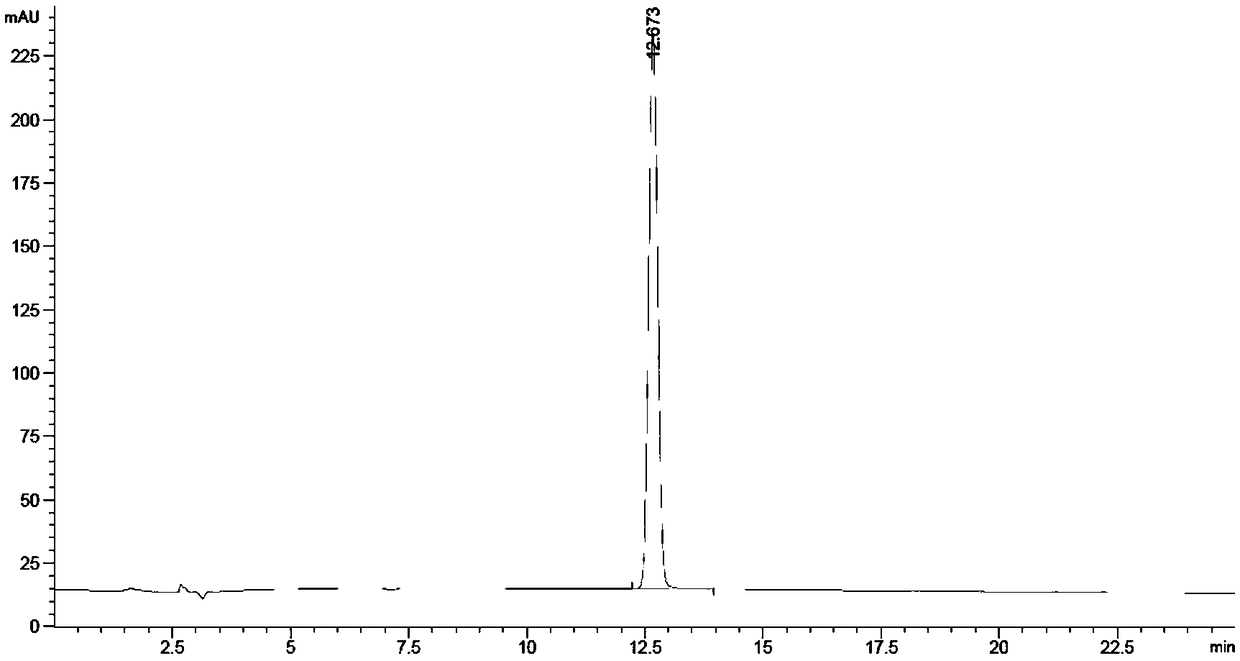
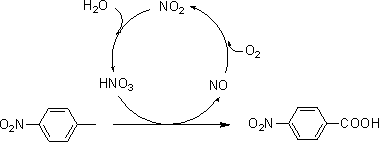
The invention relates to the field of production of chemical engineering products, in particular to a method for synthesizing p-nitrobenzoic acid. The method comprises the following steps of using p-nitrotoluene as the raw material, using diluted nitric acid as a reaction medium and a catalyst, using oxygen as an oxidant, and oxidizing in a high-pressure kettle, so as to prepare the p-nitrobenzoicacid; cooling, separating crystal form solid in the reaction liquid, sucking and filtering, so as to obtain a mother liquid and filter cake; spraying the filter cake by water, and drying, so as to obtain a light yellow product, namely p-nitrobenzoic acid. The method for synthesizing the p-nitrobenzoic acid has the advantages that the production purity is high, the preparation is simple, the costis low, the pollution is avoided, and the green effect is realized.
Owner:浙江优创材料科技股份有限公司
Preparation method of zinc oxide nano-particles for fast detecting explosive atmosphere
ActiveCN104944463AQuick responseLow detection limitMaterial nanotechnologyZinc oxides/hydroxidesExplosive atmospheresElectronic band structure
The invention provides a preparation method of zinc oxide nano-particles for fast detecting explosive atmosphere. The method includes two steps that zinc oxide nano-sheets are prepared and the zinc oxide nano-particles are formed in the annealing treatment process. Zinc oxide prepared through a sol-gel method and transition metal doped zinc oxide are of a nano-sheet structure and a nano-particle structure, transition metal doping can change the energy band structure of the nano-sheet structure and the nano-particle structure of the zinc oxide and increase the surface defects of materials, and therefore the gas-sensitive property of the materials is improved. A zinc oxide nano-particle material obtained through the method can sensitively and fast detect the detonable atmosphere with the raw materials being ammonium nitrate, urea and various nitro-explosives such as trinitrotoluene, dinitrotoluene, nitrotoluene, picric acid and cyclonite at room temperature of 25 DEG C in 2-50 seconds, responding is fast, and the detection limit is low.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Method for synthesizing p-nitrotoluene-ortho-sulfonic acid from p-nitrotoluene adopting mother liquid circulation style
InactiveCN1762992AReduce dosageEmission reductionSulfonic acid preparationP-nitrotolueneP-nitrotoluene-o-sulfonic acid
The present invention discloses mother liquid circulating p-nitrotoluene sulfonating process of preparing p-nitrotoluene sulfonic acid. The process includes the following steps: adding fuming sulfuric acid in 20 % into molten p-nitrotoluene, sulfonation, and maintaining the temperature until completing reaction; cooling to crystallize, filtering to obtain sulfuric acid mother liquid; mixing molten p-nitrotoluene and sulfuric acid mother liquid, dropping fuming sulfuric acid in 50-65 %, sulfonation, and maintaining the temperature until completing reaction; cooling, crystallizing, filtering to obtain filter cake as NTS and filtrate as sulfuric acid mother liquid; reusing parital sulfuric acid mother liquid, adding water into residual filtrate to regulate sulfuric acid concentration, and filtering to obtain NTS crystal; and circulating so on. The present invention adopts fuming sulfuric acid as sulfonating agent, circulating partial sulfuric acid mother liquid, and has the advantages of reduced sulfuric acid consumption and decreased waste acid exhaust.
Owner:TIANJIN UNIV
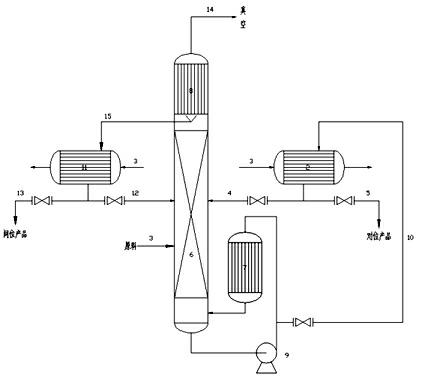
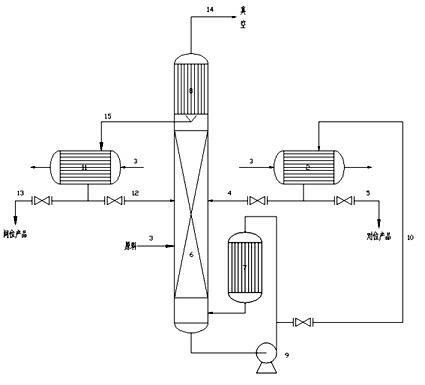
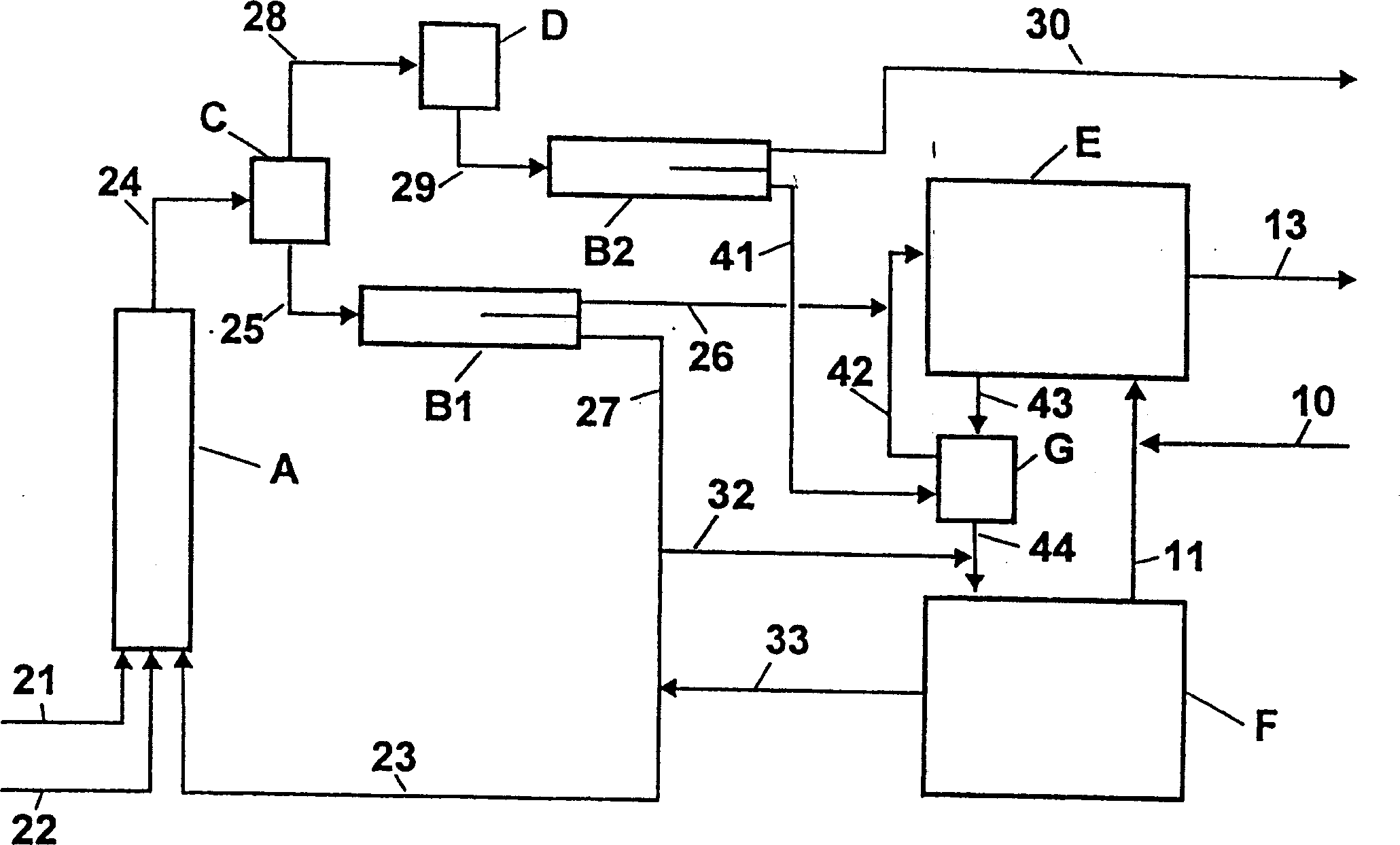
Device and method for preparing high purity m/p-nitrotoluene by coupling rectification and crystallization
InactiveCN102126958ASimple structureReasonable workmanshipOrganic chemistryOrganic compound preparationReboilerP-nitrotoluene
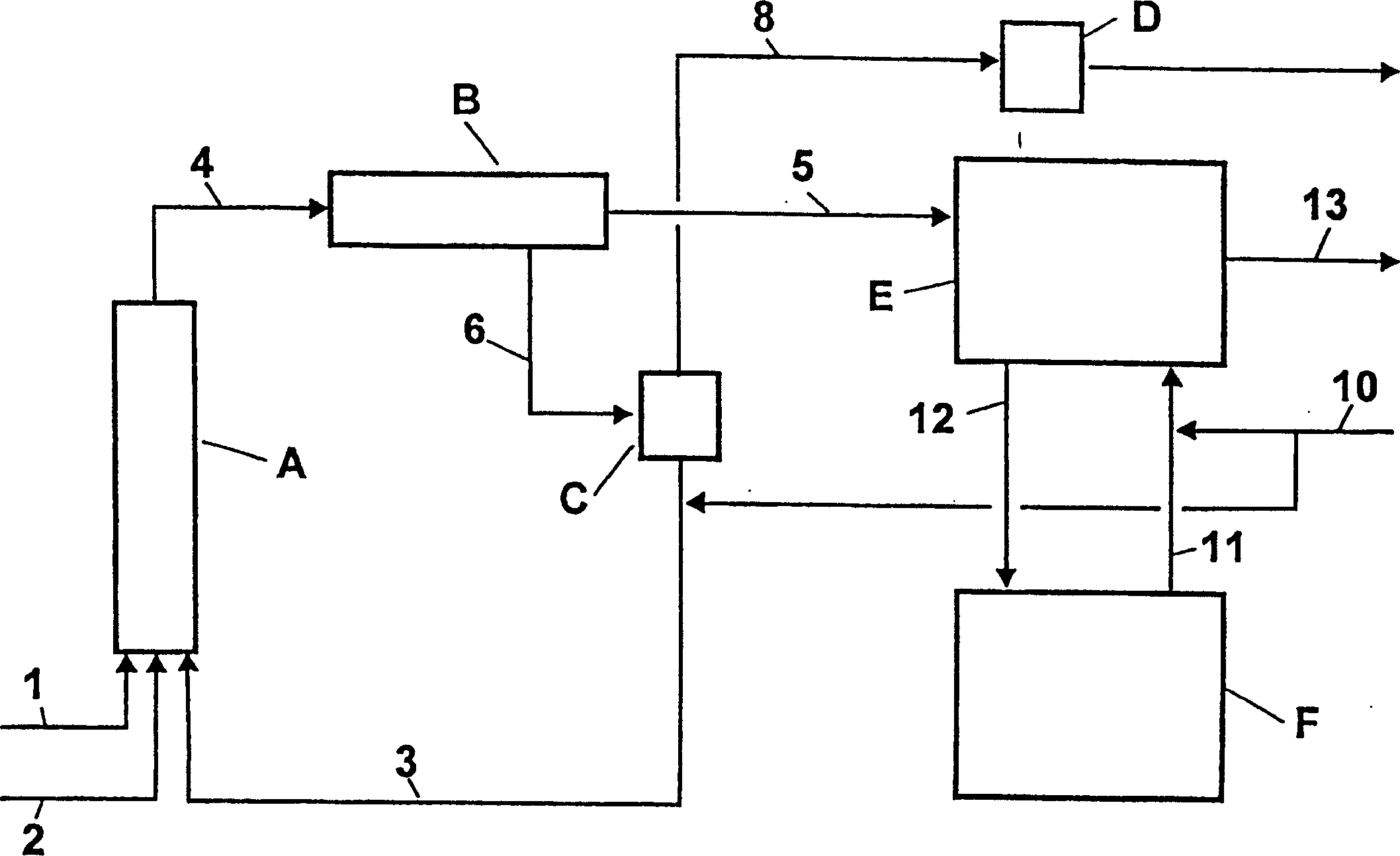
The invention discloses a device and method for preparing high purity m / p-nitrotoluene by coupling rectification and crystallization. In the device disclosed by the invention, a negative pressure rectifying tower (6) is connected with a raw material pipeline (1), a condenser (8) is arranged in the top of the negative pressure rectifying tower (6), the bottom of the condenser (8) is connected with an m-nitrotoluene crystallizer (11) through an m-nitrotoluene enriching pipeline (15), an m-nitrotoluene mother liquor (12) is connected with the negative pressure rectifying tower (6), the negative pressure rectifying tower (6) is connected with a vacuum pump (14) through a negative pressure pipeline (14), the bottom of the negative pressure rectifying tower (6) is connected with a falling film reboiler (7) and a circulating pump (9) through a pipeline, the falling film reboiler (7) is connected with a p-nitrotoluene crystallizer (2) through a p-nitrotoluene enriching pipeline (10), and a p-nitrotoluene mother liquor pipeline (4) is communicated with the negative pressure rectifying tower (6). The device disclosed by the invention has reasonable structure, simple process and low comprehensive energy consumption, is convenient in operation and is an ideal preparation device of high purity m / p-nitrotoluene.
Owner:JIANGSU HUAIHE CHEM
Aramid fiber-ultrahigh molecular weight polyethylene composite sheet material processing method
ActiveCN104960306AImprove anti-agingHigh bonding strengthLamination ancillary operationsNon-macromolecular adhesive additivesEpoxyMetasilicate
The invention discloses an aramid fiber-ultrahigh molecular weight polyethylene composite sheet material processing method. The method comprises fiber impregnation, single fiber layer hot pressing and multiple composite layer hot pressing. First glue comprises a styrene-vinyl acetate copolymer, epoxy resin, terpene resin, polyethylene glycol, p-nitrotoluene and strontium fluoride. Second glue comprises a propylene-vinyl acetate copolymer, polystyrene, terpene resin, polyethylene glycol, 2,4-dimethylphenol and potassium metasilicate. Through improvement of the glue and use of a multi-ingredient composite formula, bonding strength and density of the glue to aramid fibers and polyethylene fibers are effectively improved, and composite sheet material toughness and shock resistance are improved. Through freezing and unfreezing of the fibers subjected to impregnation, glue inner stress and surface tension are effectively reduced. Through UV treatment on the fibers subjected to impregnation, glue and fiber bonding strength is improved and a composite sheet material service life is prolonged.
Owner:CHANGSHU YONGLIJIAN NEW MATERIALS
Method for preparing dinitrotoluene
ActiveUS20100145109A1Easy to separateReduce excessChemical/physical/physico-chemical processesChemical recyclingP-nitrotolueneNitration
The present invention relates to a process for preparing dinitrotoluene. The process of the invention for preparing dinitrotoluene from mononitrotoluene, which comprises carrying out a mononitrotoluene nitration reaction using a nitrating mixture comprising nitric acid, sulphuric acid and water resulting in a two-phase medium and separating the organic and aqueous phases of said two-phase medium, is characterized in that the mononitrotoluene nitration is carried out using a nitrating mixture comprising at most 10% by weight of water resulting in a two-phase medium, in that the organic and aqueous phases of said two-phase medium are separated, and in that the aqueous phase derived from the separating operation is recycled, at the end of the mononitrotoluene nitration reaction and before the separation of the organic and aqueous phases, such that the weight ratio of the aqueous phase to the organic phase is at least equal to 1.2.
Owner:VENCOREX FRANCE
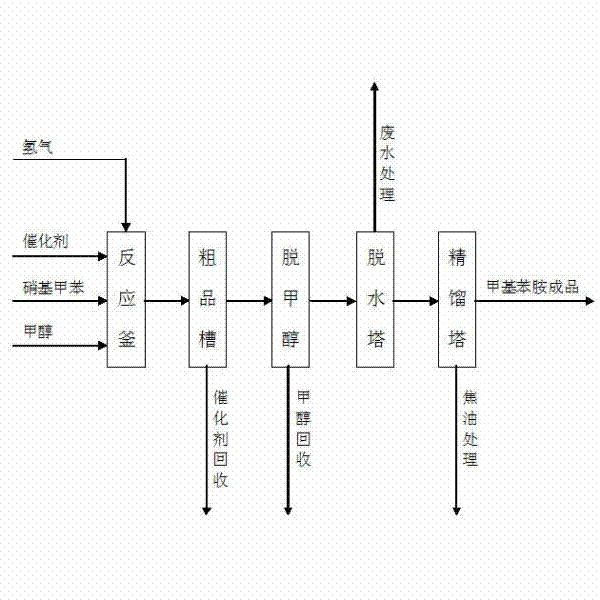
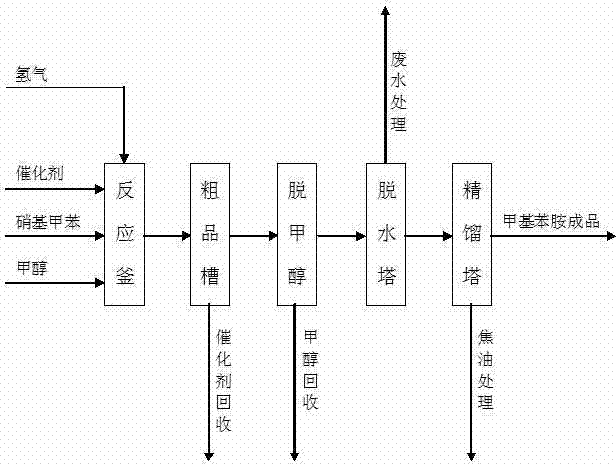
Method for producing methylaniline from nitrotoluene through liquid-phase hydrogenation reduction
InactiveCN102399154AReduce control requirementsReduce energy consumptionOrganic compound preparationAmino compound preparationMethylanilineAutomatic control
The invention discloses a method used for producing methylaniline from nitrotoluene through liquid-phase hydrogenation reduction. The production method of methylaniline comprises steps of feeding reduction and pressure-reducing separation. In the feeding reduction process, methanol, a Raney nickel catalyst, hydrogen and nitrotoluene are added to a reaction vessel; under the existence of both the solvent methanol and the catalyst, a liquid-phase catalyzed hydrogenation reaction is carried out, such that nitrotoluene is produced. According to the invention, the controlling requirements of production indexes such as temperature and pressure are low, and the method is advantaged in easy operation and management, high equipment automatic control level, good product quality, high yield, low energy consumption, recoverable catalyst and methanol, low wastewater generation, low tar, good environmental benefit, low operation cost, and high adaptability. The method can be used in reduction reactions of o-, m- or p-nitrotoluene for producing o-, m- or p-methylaniline.
Owner:JIANGSU FIRST CHEM MFG
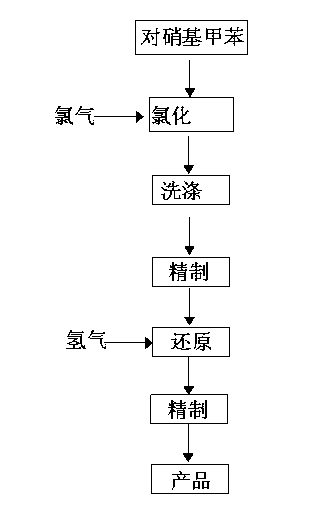
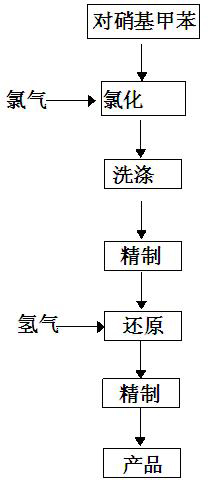
A method for preparing 3-chloro-4-methylaniline
InactiveCN102701996AReduce usageReduce lossOrganic compound preparationAmino compound preparationMethylanilinePtru catalyst
The invention discloses a method for preparing 3-chloro-4-methylaniline. The method comprises the following steps: 0.48-0.54 weight parts of chlorine is pumped into paranitrotoluene for chlorination in a chlorine kettle under the condition of stirring temperature and stirred for 10 minutes, then materials in the chlorine kettle are washed in a washing kettle; the washed materials are transferred into a refining kettle to refine, then the refined materials are transferred into a reduction tower with a catalyst, and 0.038-0.044 weight parts of hydrogen is pumped into the reduction tower for reduction; and the reduced materials are transferred into the the refining kettle to repurify to obtain the final product. The method for preparing 3-chloro-4-methylaniline provided by the invention saves the usage of raw materials effectively and reduces energy consumption rationally.
Owner:WUJIANG TUNCUN PIGMENT PLANT
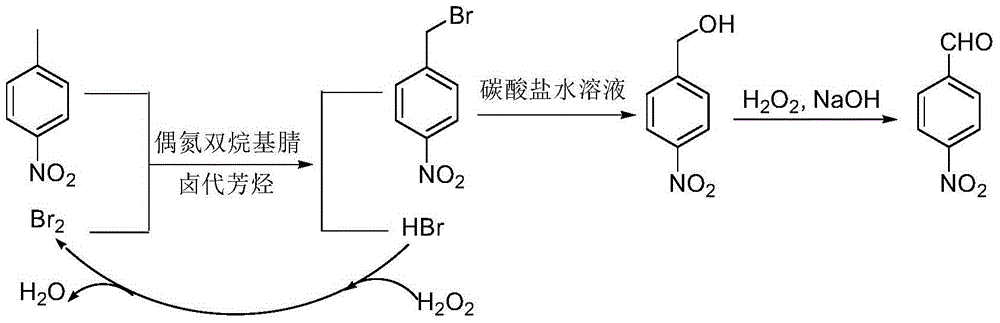
Preparation method of p-nitrobenzaldehyde
InactiveCN105348107AEasy to cleanReduce pollutionOrganic chemistryOrganic compound preparationP-nitrotolueneSolvent
The invention discloses a preparation method of p-nitrobenzaldehyde. The preparation method comprises the following steps: p-nitrotoluene, which is used as a raw material, undergoes bromine bromination under the catalysis of azo-dialkyl nitrile, so as to generate 4-nitrobenzyl bromide and hydrogen bromide; hydrolysis of 4-nitrobenzyl bromide is catalyzed by an aqueous carbonate solution to obtain p-nitrobenzyl alcohol; and hydrogen peroxide oxidation of p-nitrobenzyl alcohol is catalyzed by sodium hydroxide so as to generate a target product, namely p-nitrobenzaldehyde. According to the invention, the azo-dialkyl nitrile solid catalyst replaces a peroxycarbonate liquid phase catalyst to catalyze the bromination reaction, so as to raise operational safety of the industrial preparation reaction; by using aryl halide as a solvent medium, use of a haloalkane solvent medium is avoided, and pollution of volatile organic solvents with low boiling point is avoided; and by the hydrogen peroxide oxidation method, cleanliness of the industrial preparation reaction is raised, and environmental pollution is reduced. According to the invention, product yield is increased; yield is raised by about 3% in comparison with yield of existing traditional industrial methods; overall yield reaches 76%; and product purity reaches 99% and above.
Owner:NANJING UNIV OF SCI & TECH
P-nitrotoluene hydrogenated ruthenium-lanthanum double metal catalyst and preparation method thereof
InactiveCN106955729AHigh catalytic activityHigh selectivityMolecular sieve catalystsOrganic compound preparationHydrogenP-nitrotoluene
The invention relates to a p-nitrotoluene hydrogenated ruthenium-lanthanum double metal catalyst and a preparation method thereof. The catalyst comprises an active ingredient ruthenium, an auxiliary agent lanthanum and a carrier. The catalyst is shown by x%Ru-y%La / carrier, in which x% represents percentage by mass of Ru in the catalyst, y% represents percentage by mass of La in the catalyst, and the catalyst comprises the following components in percentages by mass: 0.5-5% of Ru, 0.2-2% of La and the balance of carrier. The precursor of the catalyst is prepared by means of an impregnating-precipitating method and is reduced in a hydrogen-containing atmosphere to obtain the p-nitrotoluene hydrogenated ruthenium-lanthanum double metal catalyst. In a reaction of preparing p-methyl-cyclohexylamine by p-nitrotoluene hydrogenation, p-methyl-cyclohexylamine will be strongly adsorbed on the catalyst so as to generate a lot of tar to inhibit the reaction; and by adding lanthanum, the problem that the catalyst strongly adsorb p-methyl-cyclohexylamine is solved well, and the generating amount of tar is reduced.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
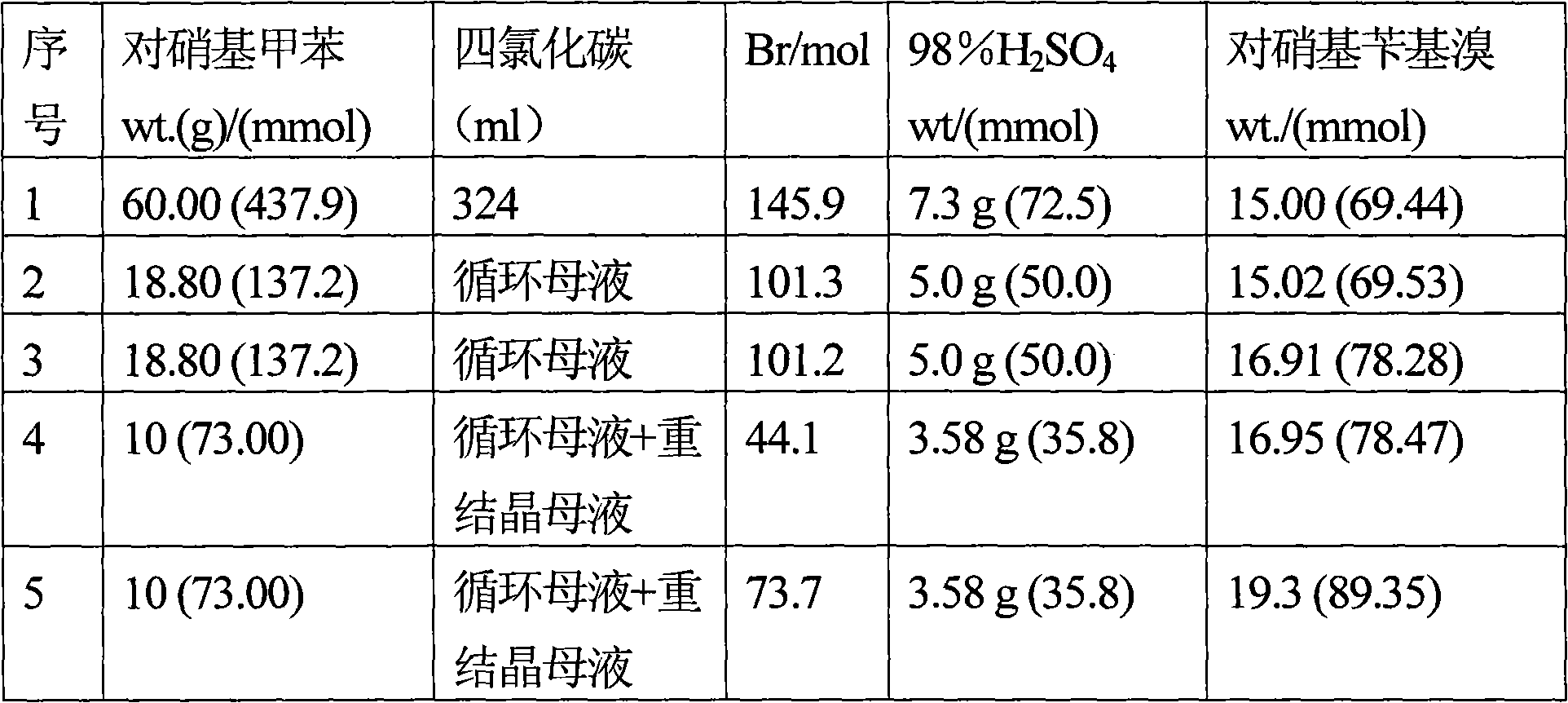
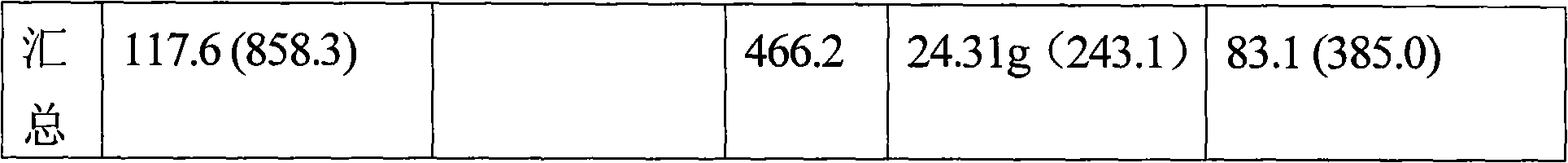
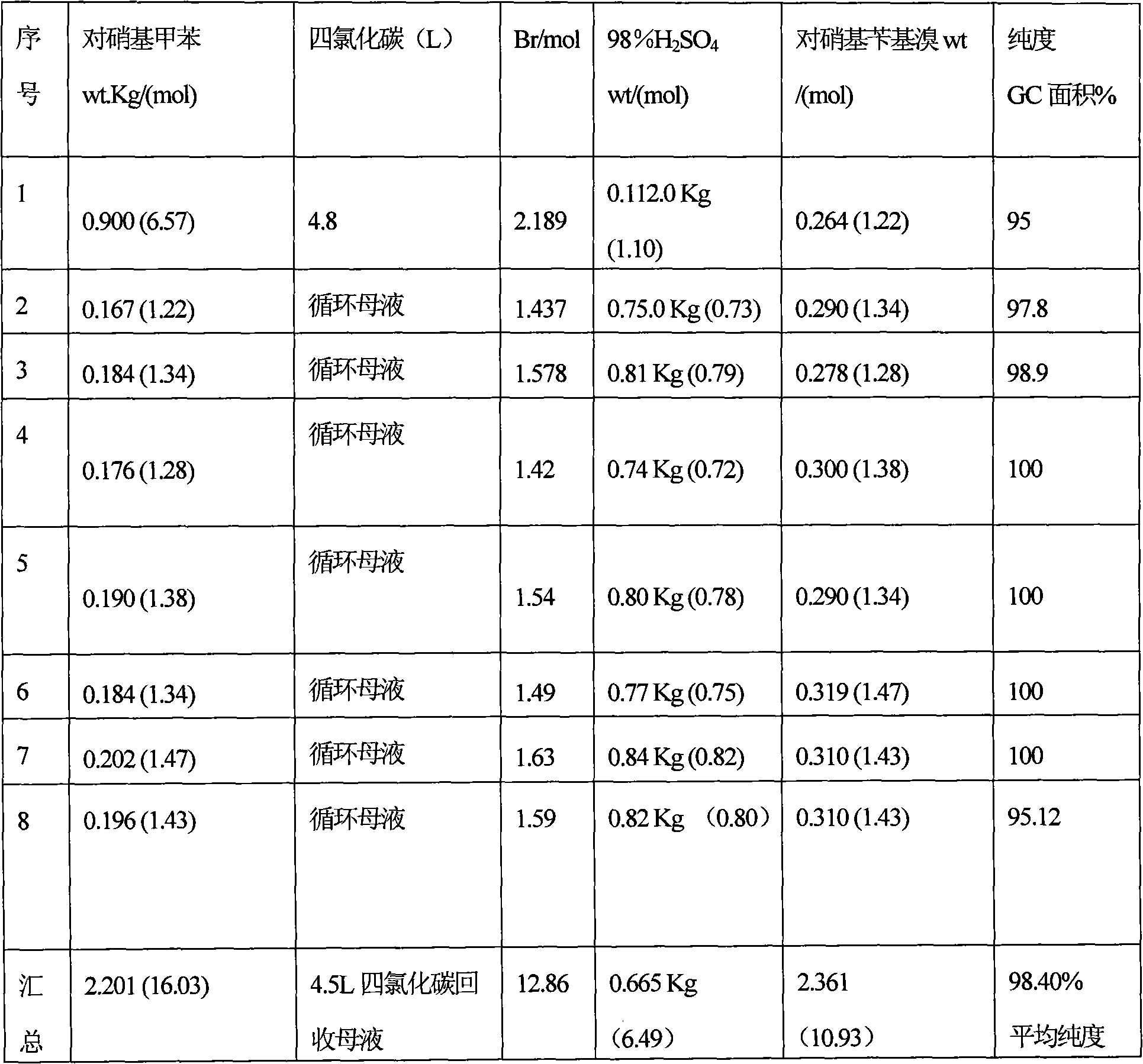
An improved process for the preparation of para-nitrobenzyl bromide
InactiveCN102307844AHigh purityAvoiding the Problem of Disposing of Organic WasteOrganic chemistryOrganic compound preparationP-nitrotolueneSolvent
An improved process for the preparation of p-nitrobenzyl bromide from p-nitrotoluene is disclosed wherein large excess of the reactant in carbon tetrachloride is treated with 2:1 bromide-bromate reagent and the product is isolated efficiently and with high purity through selective cold crystallization from reaction mass so as to allow the mother liquor to be recycled in a subsequent batch without any further work up. The cycles are continued so long as product of desired quality is obtained in adequate yield. Thereafter, the solvent and p-nitrotoluene are recovered from the mother liquor and the residue is treated with sodium borohydride to convert impurities back into reactant or product which is then recycled in the process thereby circumventing the problem of waste disposal and simultaneously yielding the desired product with >95 % yield with respect to p-nitrotoluene and >88 % bromine atom efficiency.
Owner:COUNCIL OF SCI & IND RES
P-nitrotoluene-2-sulfonic acid continuous synthesis method
ActiveCN108586296ALow mass transfer efficiencyReduce heat transfer efficiencySulfonic acid preparationSynthesis methodsP-nitrotoluene
Disclosed is a p-nitrotoluene-2-sulfonic acid continuous synthesis method. The p-nitrotoluene-2-sulfonic acid continuous synthesis method comprises melting or dissolving p-nitrotoluene in organic solvent or inorganic solvent, and inletting the melt of solution together with sulfur trioxide mixed gas into a film sulfonation reactor for sulfonation reaction, filtering liquid reaction products containing the inorganic solvent to remove the inorganic solvent and then feeding the reaction products into an ageing tank, or directly feeding the solvent-free or organic solvent-containing liquid reaction products into the ageing tank for stirred ageing; and performing hydrolysis by adding in deionized water to obtain hydrolyzed products, which, if containing no organic solvent, are p-nitrotoluene-2-sulfonic acid, else, performing extraction by adding in deionized water for dissolution in aqueous phase to obtain the p-nitrotoluene-2-sulfonic acid. The p-nitrotoluene-2-sulfonic acid continuous synthesis method has the advantages of being low in production cost, free from producing waste acid, high in safety performance and capable of achieving continuous production.
Owner:CHINA RES INST OF DAILY CHEM IND
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1271040CNo pollution in the processLow costOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
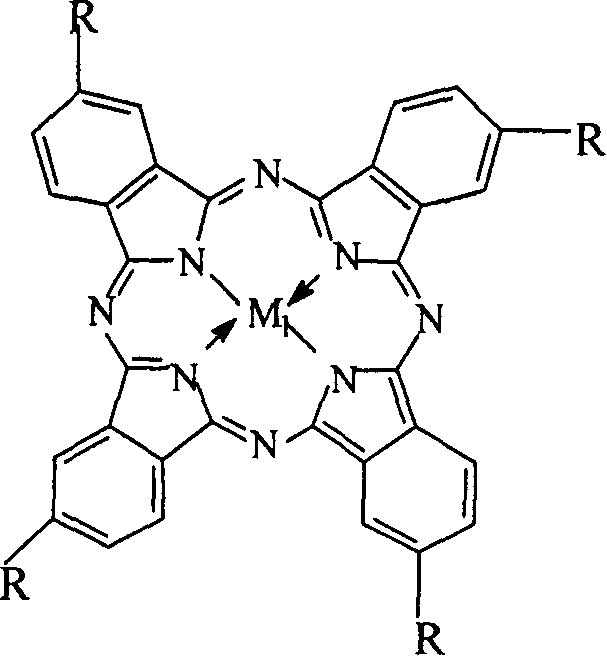
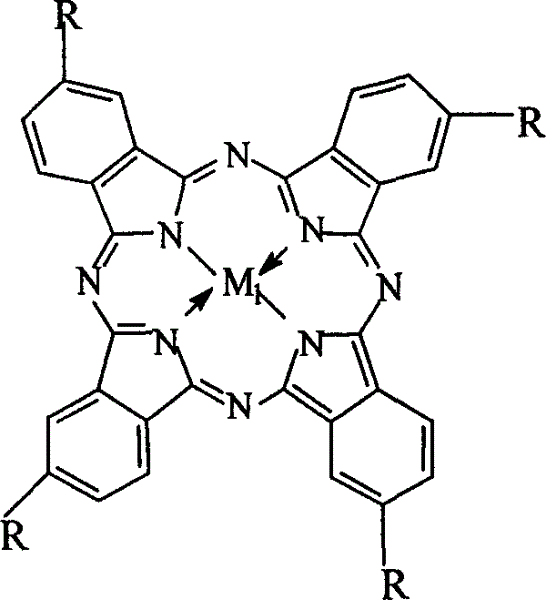
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Method for removing nitrotoluene in high-concentration waste acid
InactiveCN102557179ASimplified processing stepsGood processing effectWater contaminantsWater/sewage treatment by extractionSolubilityHigh concentration
The invention relates to a method for removing nitrotoluene in high-concentration waste acid. The method comprises adding an extractant into the high-concentration waste acid at a certain temperature, extracting, and separating the extractant from the waste acid; and recovering the extractant. Compared with the prior art, the invention has significant advantages that the invention has simple treatment steps, significant treatment effect and mononitrotoluene (MNT) and dinitrotoluene (DNT) removal rate up to above 99%, the treated high-concentration waste acid has high purity and can be directly used for other chemical processes, and the invention is suitable for industrialization; a perfluoro solvent serving as the extractant has good fluidity, chemical inertness and good phase separation performance, and has solubility for nitrotoluene compounds at a temperature higher than a critical temperature, thus it can be reutilized and maintains high removal rate; the invention directly treats the high-concentration waste acid without diluting with water, avoids subsequent wastewater treatment, and lowers the cost; and the inventive method has few apparatus, low manufacture cost, low investment and low running cost.
Owner:NANJING UNIV OF SCI & TECH +1
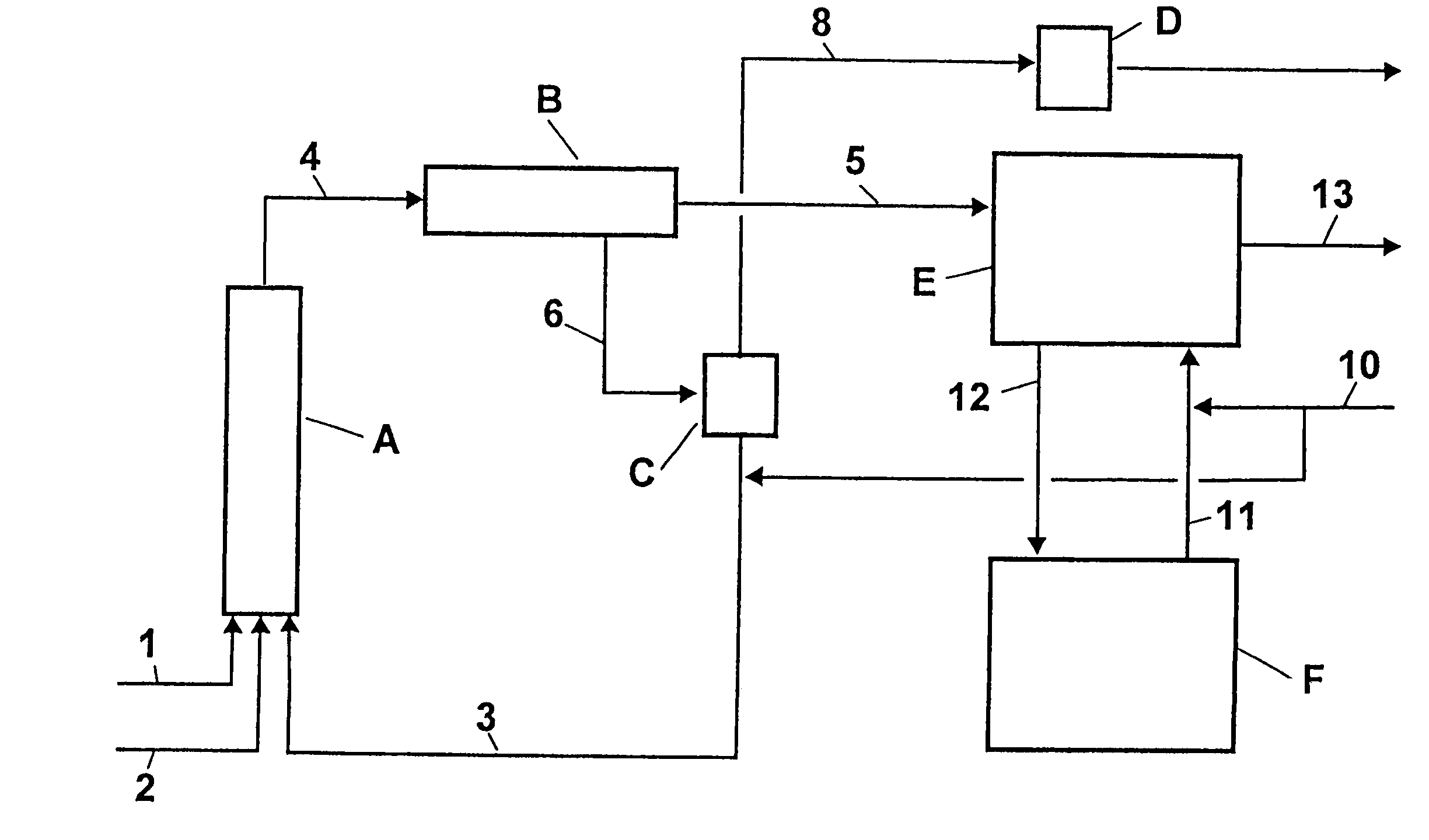
Two step preparation of dinitrotoluene method
Two-stage nitration of toluene to produce dinitrotoluene involves a first adiabatic stage and a second isothermal stage. Two-stage nitration of toluene to produce dinitrotoluene comprises (1) adiabatically reacting toluene with nitrating acid such that the toluene is at least 90 % reacted and a maximum of 50 % is reacted to dinitrotoluene, separating the mononitrotoluene-containing organic phase and the aqueous sulfuric acid-containing phase, concentrating the acid aqueous phase by flash evaporation and recycling the concentrated sulfuric acid to the first or second stage or to a concentration process in the second stage; and (2) isothermally completing the nitration of the mononitrotoluene-containing organic phase, separating the organic and aqueous sulfuric acid-containing phases, concentrating the acid, aqueous phase by vacuum evaporation and recycling the acid to the first or second stage.
Owner:COVESTRO DEUTSCHLAND AG
Production method of p-toluidine
InactiveCN102180801AReduce manufacturing costLow reaction temperatureOrganic compound preparationAmino compound preparationPalladium on carbonFiltration
The invention provides a production method of p-toluidine and relates to the technical field of chemical production. In the production method, liquid phase hydrogenation reduction is directly carried out on p-nitrotoluene in the presence of a palladium-carbon catalyst so as to produce the p-toluidine. The production method has the advantages of low reaction temperature, low reaction pressure and high reaction conversion rate; and the product performs water distribution and crystallization after hermetic filtration, the yield of the product is up to 98.3%, and no three wastes are basically emitted. Because no other byproducts are generated in the production process of the produce, the purity of the product is up to 98.6%, and the next generation products can be directly produced.
Owner:GAOYOU AUXILIARY FACTORY
Method for synthesizing o-chloro-p-aminotoluene from o-chloro-p-nitrotoluene through catalytic hydrogenation
InactiveCN109053461AThe process is simple and controllableReduce manufacturing costOrganic compound preparationAmino compound preparationDistillationP-nitrotoluene
The invention discloses a method for synthesizing o-chloro-p-aminotoluene from o-chloro-p-nitrotoluene through catalytic hydrogenation. The method comprises the following steps: enabling the o-chloro-p-nitrotoluene to be dissolved in a methyl alcohol solvent according to a mass ratio of 1 to 1, and adding a raney nickel catalyst; after dissolving, feeding hydrogen, wherein a molar weight proportion of the o-chloro-p-nitrotoluene to the hydrogen is 1 to (3.5 to 4), controlling a reaction temperature to be 90-95 DEG C, and controlling a reaction pressure to be 1.6-2.2 MPa; while an input amountof the o-chloro-p-nitrotoluene is 1 ton, reducing reaction time is about 5-6 hours; transferring reducing solution to a solvent distillation column, performing atmospheric distillation to obtain coarse o-chloro-p-aminotoluene; transferring the coarse o-chloro-p-aminotoluene to a rectifying tower, performing negative pressure distillation to obtain refined o-chloro-p-aminotoluene, wherein a purityof the o-chloro-p-aminotoluene measured by using a gas chromatographic method is greater than 98.00%. Multi-sulfur waste water and multi-sulfur waste residues are not generated in a production process, the method is energy-saving and environmentally friendly, an operating process of the technology is simple and controllable, and production cost is reduced.
Owner:宜宾市南溪区红源化工有限公司
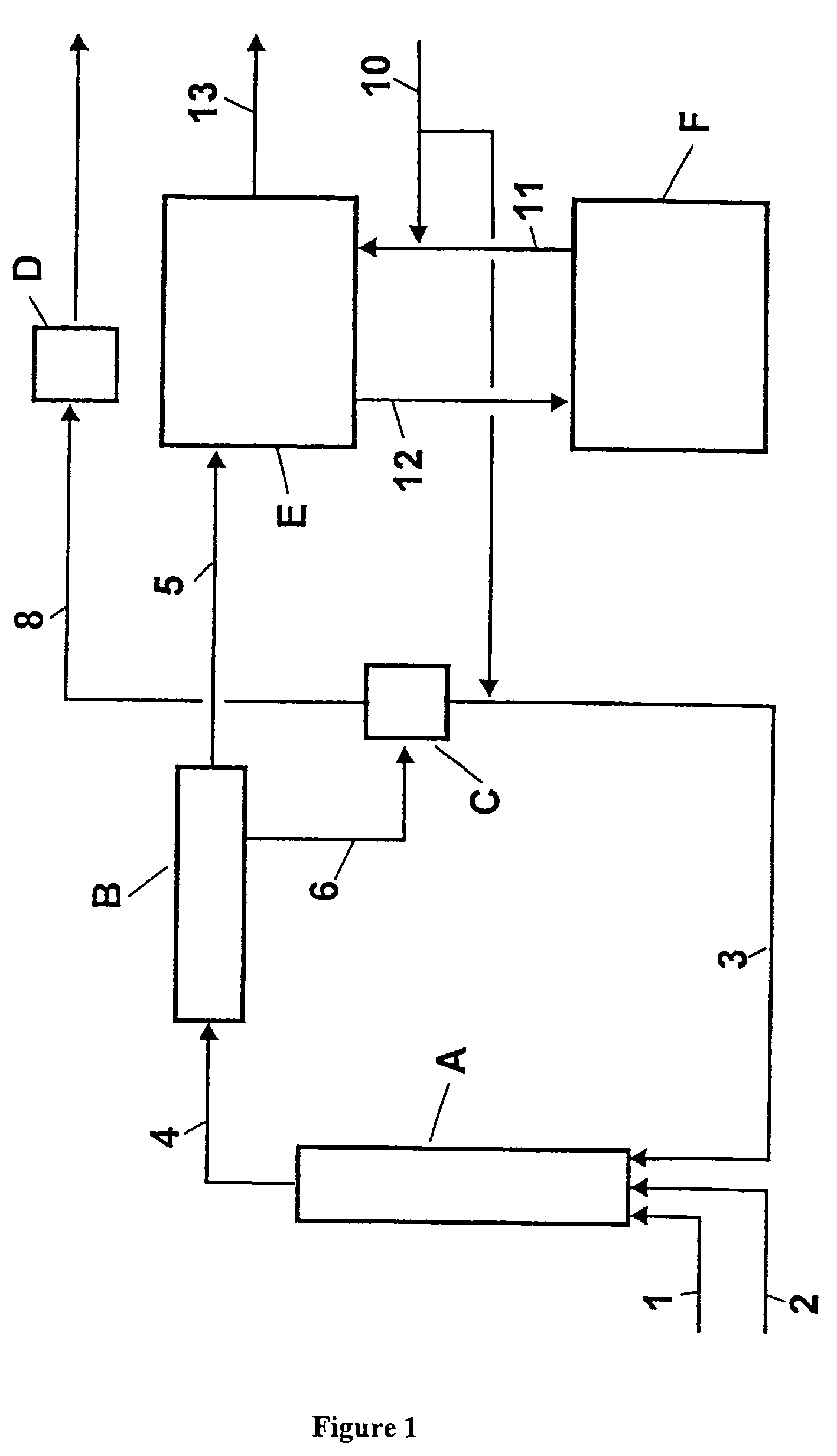
Process for the two-step production of dinitrotoluene
The invention relates to a process for the production of dinitrotoluene by the two-stage nitration of toluene. In the first stage of this process, toluene was reacted adiabatically with nitrating acid so that at least 90% of the toluene was reacted off and no more than 70% of the toluene formed dinitrotoluene. The resulting organic phase containing mononitrotoluene and the aqueous acid phase containing sulfuric acid were separated, and the aqueous acid phase containing sulfuric acid was concentrated by flash evaporation. The resulting concentrated sulfuric acid was recycled into the reaction in the first stage, and / or into the reaction in the second stage, and / or into the concentration in the second stage.In the second stage, the organic phase containing mononitrotoluene from the first stage was completely reacted isothermally with nitrating acid. The organic phase and the aqueous acid phase containing sulfuric acid were then separated, and the aqueous acid phase containing sulfuric acid was concentrated by vacuum evaporation. The resulting concentrated sulfuric acid was recycled into the reaction in the first stage and / or the second stage.
Owner:COVESTRO DEUTSCHLAND AG
Preparation method and application of schottky junction explosive atmosphere sensing material
ActiveCN105866179ALower working temperatureShort response timeMaterial analysis by electric/magnetic meansExplosive atmospheresAmmonium nitrate
The invention relates to a preparation method and application of a schottky junction explosive atmosphere sensing material. According to the method, a schottky junction is constructed through a silicon-zinc oxide nuclear shell nanowire array and a nanowire array top graphene top electrode; graphene and the silicon-zinc oxide nuclear shell nanowire array are utilized for forming the schottky junction, the purposes of increasing adsorption sites, improving explosive adsorption energy and adjusting the response magnitude. The sensing material obtained through the method is the silicon-zinc oxide nuclear shell nanowire array / graphene schottky junction. The sensing material is good in response to room temperature steam of eight types of explosives including trinitrotoluene (TNT), dinitrotoluene (DNT), paranitrotoluene (PNT), picric acid (PA), hexogen (RDX), urea, black powder (BP) and ammonium nitrate (AN); the response time and recovery time are short and shorter than 5 seconds.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Production method for 2-chloro-4-methyl nitrobenzene
InactiveCN103435491AHigh yieldQuality improvementOrganic chemistryOrganic compound preparationMolecular sieveNitrobenzene
The invention relates to a production method for 2-chloro-4-methyl nitrobenzene. The method comprises the following steps: using p-nitrotoluene as a raw material and 4A type or 13X type zeolite molecular sieve as a catalyst to perform chlorination reaction with chlorine gas of which molar weight is the same as those of the p-nitrotoluene and the 4A type or 13X type zeolite molecular sieve, and rectifying a chlorination solution by vacuum rectification to obtain a 2-chloro-4-methyl nitrobenzene product. According to the method, wastewater cannot be produced in a production process, so that the clean production of the 2-chloro-4-methyl nitrobenzene is achieved, the product yield is increased, the product quality is improved, the yield can reach more than 95%, and the content can reach more than 99%.
Owner:淮安嘉诚高新化工股份有限公司
Treatment method of residues from nitrotoluene rectification kettle
InactiveCN103772208ASimple processShort processOrganic compound preparationAmino compound preparationMethylanilineP-nitrotoluene
The invention discloses a treatment method of residues from a nitrotoluene rectification kettle. The method comprises the following steps: performing a hydrogenation reduction reaction on the residues from the rectification kettle and hydrogen in the presence of a catalyst, thereby generating methylaniline comprising three isomers, namely, o-toluidine, m-toluidine and p-toluidine, from the mononitrotoluene in the kettle residues and generating diaminotoluene comprising two isomerides, namely, 2,4-diaminotoluene and 2,6-diaminotoluene, from the dinitrotoluene in the kettle residues; and then performing rectification separation, thereby obtaining a methylaniline finished-product and a diaminotoluene finished-product. The method is simple in process, short in procedure, clean and environment-friendly, and realizes the rational utilization of waste resources, i.e., the products with high additional values are produced by utilizing low-value resources, thereby bringing economic benefits to enterprises. As a result, the recycling of waste materials, the energy conservation and the emission reduction are realized.
Owner:淮安嘉诚高新化工股份有限公司
Preparation method of 4-amino-2-sulfonic benzoic acid
The invention discloses a preparation method of 4-amino-2-sulfonic benzoic acid, and belongs to the preparation technology of aromatic sulfonic acid. The process of the method comprises the following steps: dissolving raw materials of p-nitrotoluene o-sodium sulfonate, 4-nitro-2- sodium sulfonate benzaldehyde, 4,4'-dinitro diphenylethane-2,2'-disodium disulfonate or 4,4'-dinitro diphenylethene-2,2'-disodium disulfonate in a sodium hydroxide solution, performing oxidation with pressurized air, performing reduction with iron powder, performing filtration and crystallization to obtain 4-amino-2-sulfonic benzoic acid. The invention has a simple process, high yield and purity, and the product 4-amino-2-sulfonic benzoic acid can be prepared into p-aminosalicylic acid through simple alkali fusion technology with high yield and high product purity.
Owner:TIANJIN UNIV +1
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546458AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationPorphyrinCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Production process of p-nitrobenzaldehyde
InactiveCN102329235ASolve environmental problemsReduce the impactOrganic chemistryOrganic compound preparationP-nitrotolueneSolvent
The invention relates to a production process of p-nitrobenzaldehyde. The production process is characterized in that p-nitrobenzaldehyde is prepared by the sections of bromination, hydrolysis, oxidation, addition, alkali precipitation, refining and the like based on p-nitrotoluene as a raw material. According to the production process provided by the invention, a solvent used when bromination is carried out is dichlorethane, thereby avoiding the environmental protection problem brought by carbon tetrachloride; an unreacted raw material is used as the solvent when oxidation is carried out; when refining is carried out, 80% ethanol is used as the solvent, thereby reducing the influence of an toxic solvent on a human body; and when bromination is carried out, cheap hydrogen peroxide is added, thereby improving the utilization rate of bromine and avoiding the disadvantages of large amount of hydrogen bromide gases on environment and worker operation. Thus, the production process provided by the invention has higher environmental friendliness and safety, and is suitable for industrial production on large scale; and by test, the yield of p-nitrobenzaldehyde is above 65%, and the chromatographic purity of the finished product can reach above 99.5%.
Owner:GUANNAN YISITE CHEM
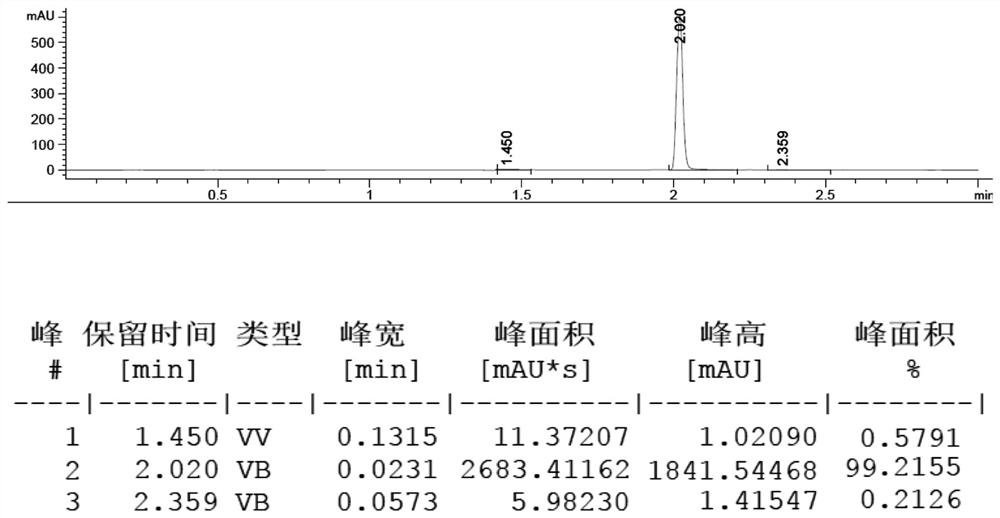
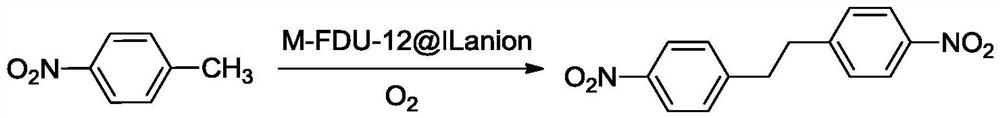
Preparation method of 4, 4'-dinitrobibenzyl
ActiveCN113636939AHigh activityImprove stabilityOrganic chemistryMolecular sieve catalystsPtru catalystP-nitrotoluene
The invention discloses a novel preparation method of 4, 4'-dinitrobibenzyl. According to the method, p-nitrotoluene is used as a reactant, oxygen is used as an oxidizing agent, ethanol is used as a solvent, transition metal doped FDU-12 mesoporous molecular sieve supported guanidine salt ionic liquid is used as a catalyst, and 4, 4 '-dinitrobibenzyl is prepared through high-selectivity oxidative dehydrogenation coupling reaction. After the reaction is finished, the catalyst phase and the product phase are easily subjected to heterogeneous separation and can be well recycled. The method has the advantages of simple operation, mild reaction conditions, small catalyst dosage, high reaction efficiency and selectivity, and small discharge of three wastes, and is a novel green and clean preparation method.
Owner:CHINA THREE GORGES UNIV
High selectivity synthesis method of p-nitrobenzaldehyde
InactiveCN102126960BReduce pollutionReduce consumptionOrganic chemistryOrganic compound preparationSynthesis methodsP-nitrotoluene
A high selectivity synthesis method of p-nitrobenzaldehyde comprises the following steps: firstly, adding p-nitrotoluene, peroxycarbonate used as catalyst and dichloroethane used as solvent in a reactor, dropping bromine at 40-50 DEG C under stirring, then reacting at 50-60 DEG C to ensure that the color of bromine fades, adding hydrogen peroxide to react at 60-70 DEG C for no less than 4 hours and prepare 4-nitrobenzyl bormide; and secondly, adding 25-35% sodium carbonate solution to hydrolyze at 80-95 DEG C and generate p-nitrobenzyl alcohol, standing to separate, and finally using oxygen as oxidant to react for no less than 25 hours in the presence of catalyst triphenylphosphine metal salt organic complex under the conditions that the temperature is 50-90 DEG C and the pressure 5.1*10<5>-1.0*10<6>. The overall yield of the method is no less than 70%, the product purity is no less than 99% and the dosage of bromine is 50-60% of the theoretical amount.
Owner:HEFEI UNIV OF TECH
Efficient cooling liquid
InactiveCN106833543AImprove cooling efficiencyReasonable ratio of ingredientsHeat-exchange elementsFiberPolyester
The invention relates to the technical field of chemical materials, in particular to efficient cooling liquid. The efficient cooling liquid is characterized in that being prepared from the following raw materials in parts by weight: 30 to 45 parts of glycerol, 20 to 28 parts of ethanol, 19 to 36 parts of propylene glycol, 8 to 14 parts of sorbitol, 3 to 10 parts of p-nitrotoluene, 2 to 10 parts of phenolic resin, 1.4 to 4.2 parts of sodium fluosilicate, 1 to 7 parts of boric sludge, 5 to 17 parts of polyester fiber, 0.1 to 0.5 part of maleic anhydride, 4 to 8 parts of aromatic amine and 3 to 15 parts of antioxidant. The efficient cooling liquid has the advantages of reasonable composition proportion, stable structure, high cooling efficiency, low production cost, safety, nontoxicity, insusceptibility to volatilization and recyclability.
Owner:安徽省东至县东鑫冲压件有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com