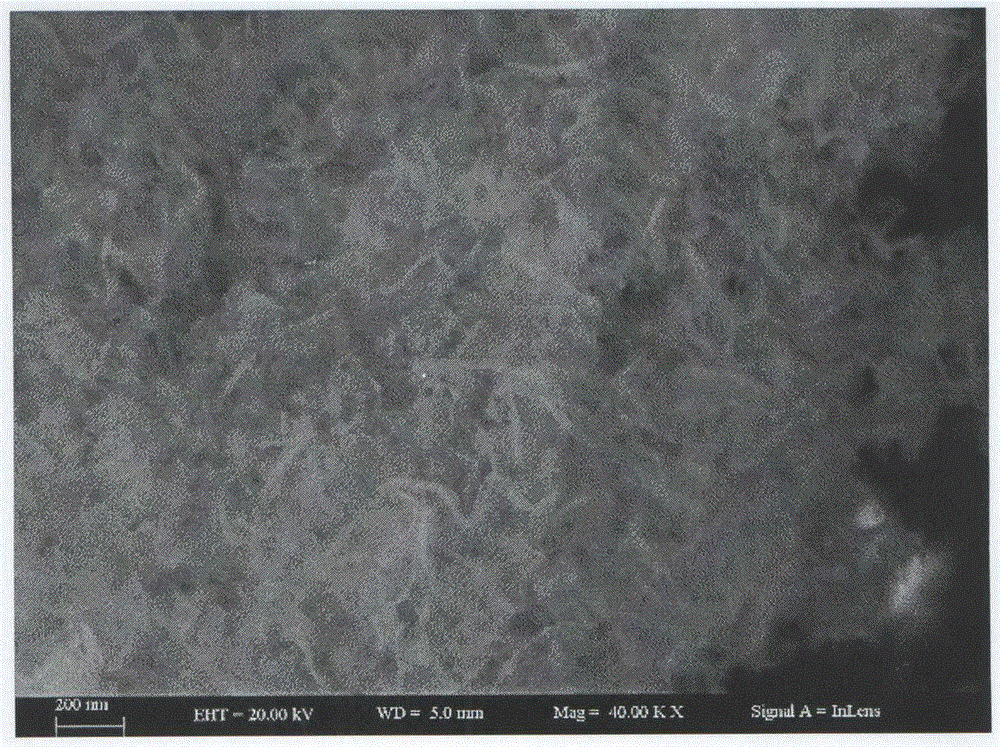
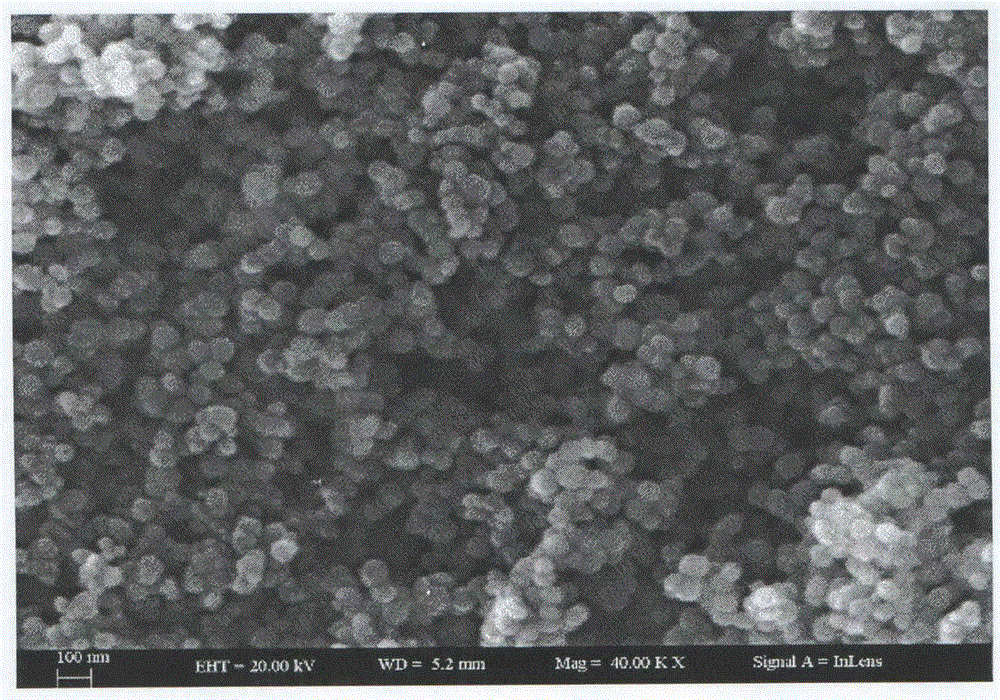
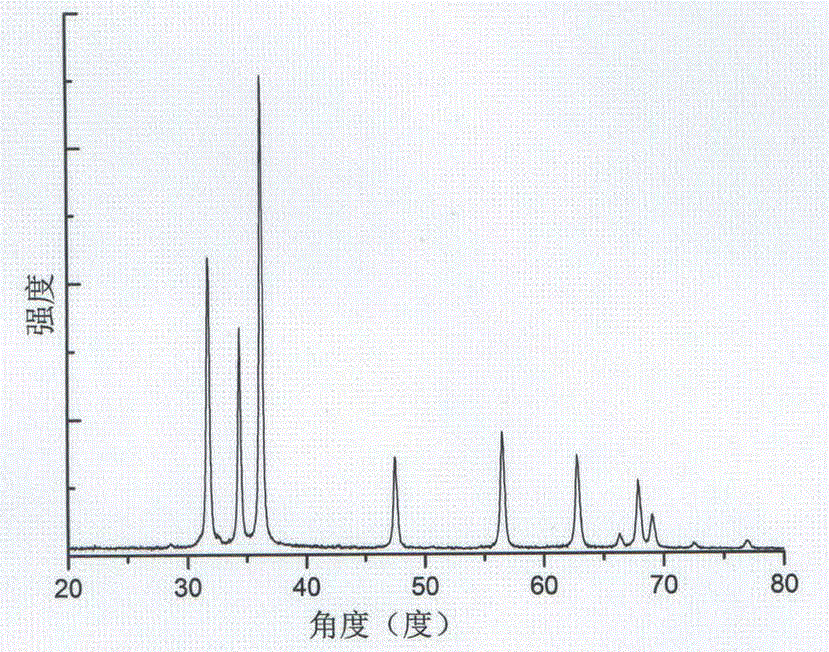
Preparation method of zinc oxide nano-particles for fast detecting explosive atmosphere
A technology of zinc oxide nano and explosives, which is applied in the field of functional materials science and gas-sensing sensing materials, can solve the problems of high power consumption, unfavorable detection, high operating temperature, etc., achieve low detection limit, fast response, and improve adsorption performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate in blue bottle A containing 50 ml of absolute ethanol;
[0039] b. Dissolve 0.4208 g of potassium hydroxide in blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0040] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until the mixture is uniform, put the solution in a water bath, control the temperature of the water bath to 60 °C, and stir for 24 hours to obtain an opaque colloid;
[0041]d, disperse the opaque colloid obtained in step c in n-heptane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, and use two washings as a washing cycle, repeat the washing cycle 3 times, The washed precipitate was transferred to a watch glass, dried in an oven at 80 ...
Embodiment 2
[0044] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate in blue bottle A containing 50 ml of absolute ethanol;
[0045] b. Dissolve 0.3000 g of sodium hydroxide in the blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0046] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until evenly mixed, put the solution in a water bath, control the temperature of the water bath to 60°C, and stir for 24 hours to obtain an opaque colloid;
[0047] d, disperse the opaque colloid obtained in step c in n-heptane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, and use two washings as a washing cycle, repeat the washing cycle 3 times, The washed precipitate was transferred to a watch glass, dried in an oven at 80 °C for 12...
Embodiment 3
[0050] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate and 0.0727 grams of nickel nitrate in blue bottle A containing 50 ml of absolute ethanol;
[0051] b. Dissolve 0.3000 g of sodium hydroxide in the blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0052] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until evenly mixed, put the solution in a water bath, control the temperature of the water bath to 25°C, and stir for 0.5 h to obtain an opaque colloid;
[0053] d. Disperse the opaque colloid obtained in step c in n-hexane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, use two washes as a wash cycle, repeat the wash cycle 3 times, and The washed precipitate was transferred to a watch glass, dried in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com