An improved process for the preparation of para-nitrobenzyl bromide
A kind of technology of p-nitrobenzyl bromide and nitrobenzyl bromide, applied in the field of improved preparation of p-nitrobenzyl bromide, can solve problems such as difficulty and no mention of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
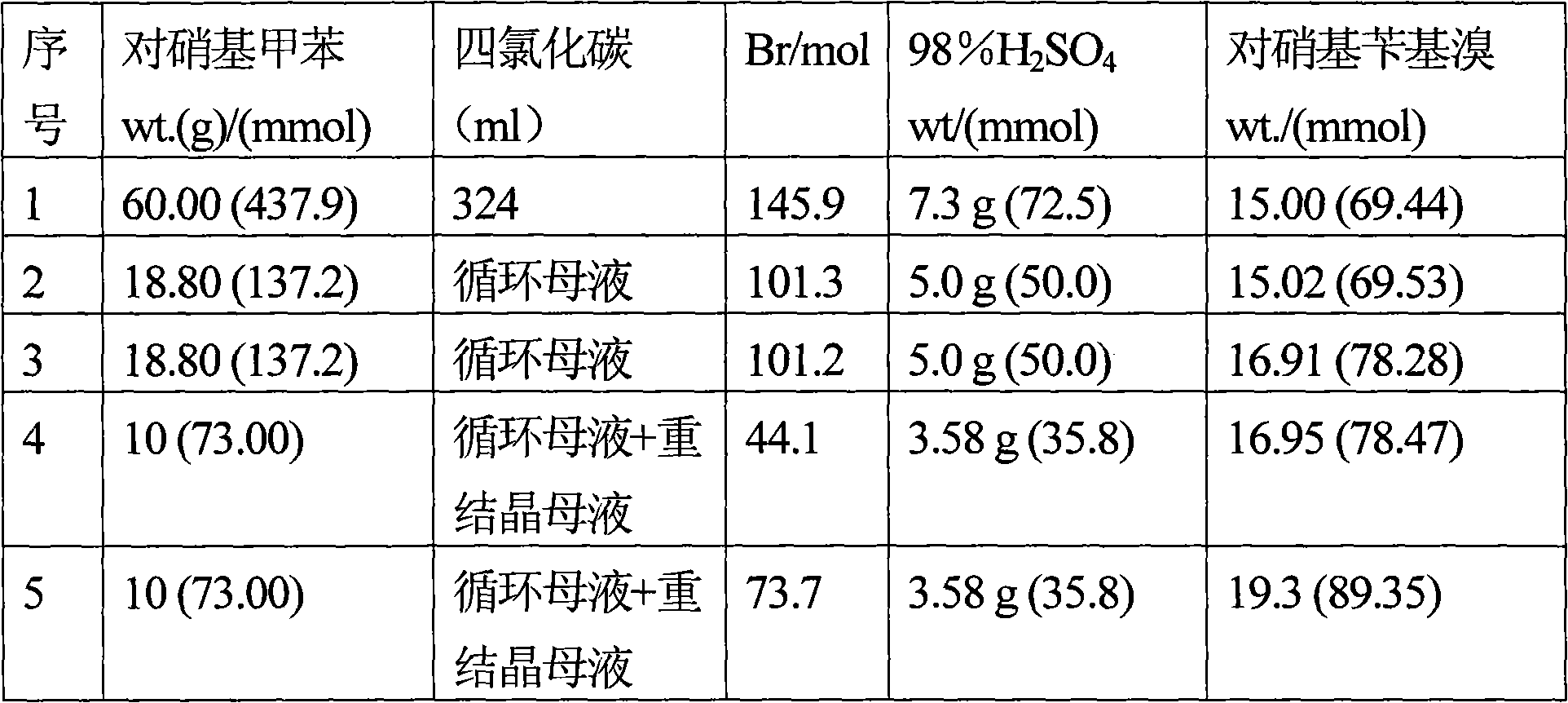
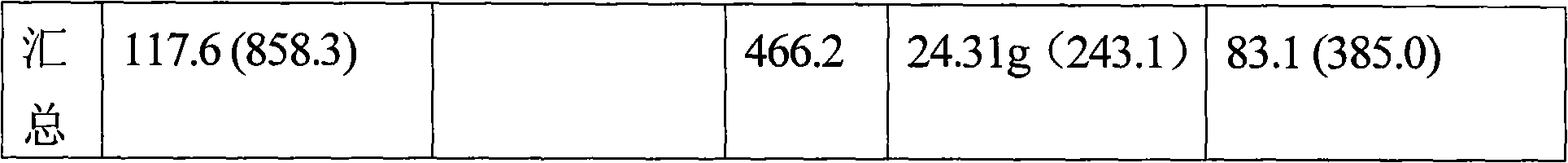
[0110] 60 grams (437.9 mmol) of p-nitrotoluene were dissolved in 324 mL of carbon tetrachloride and added to a 1.0 liter capacity glass reactor equipped with an addition funnel and reflux condenser / distillation unit assembly. Add 90 milliliters of brominating agents containing 145.9 mmol of active Br so that during the reaction the ratio of p-nitrotoluene / p-nitrobenzyl bromide ≥ 2: 1, the reaction contents are heated to reflux temperature and with a tungsten wire of 100 watts Lamp external lighting, 7.3 g of 98% H in 30 ml of water 2 SO 4 (72.5 mmole) was added to the addition funnel and the acid was added over 2 hours. (Note: If the color of the reaction mass turns tan, stop adding acid, wait for the color to disappear, and then continue adding acid.) Maintain reflux conditions for an additional 1 hour. The reaction mass was cooled to room temperature, and the organic and aqueous layers were separated. The organic phase was poured into a beaker and kept overnight in the fr...
Embodiment 2
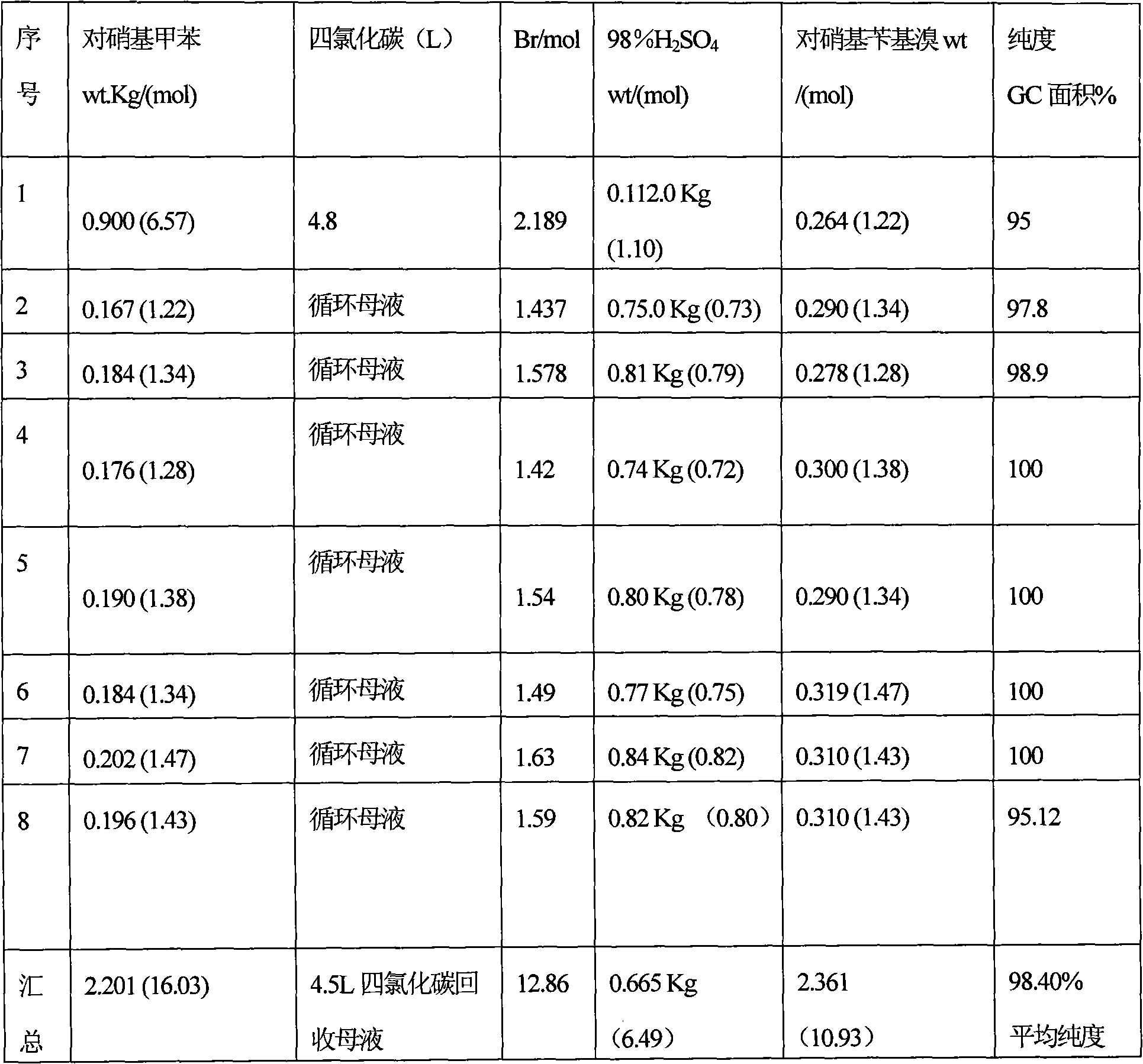
[0116] 0.9 kg (6.57 mol) of para-nitrotoluene was dissolved in 4.8 L of carbon tetrachloride and added to a 10 liter capacity glass reactor equipped with an addition funnel and reflux condenser / distillation unit assembly. Add 1.09L of brominating agent containing 2.18mol active Br so that the ratio of p-nitrotoluene to p-nitrobenzyl bromide ≥ 2:1 during the reaction, the reacted material is heated to reflux temperature and with 3 lamps of 100 watts Tungsten lamp external lighting, 112g in 0.6L water of 98% H 2 SO 4 (1.12 mmol) was added to the addition funnel and the acid was added over 2 hours. (Note: If the color of the reactant changes to tan, stop adding acid, wait for the color to disappear, and then continue adding acid). Reflux conditions were maintained for an additional 1 hour.
[0117] The reaction mass was cooled to about 40°C and the contents were poured into a separation column. The organic phase was poured into a suitable wide-mouth glass container and kept c...
Embodiment 3
[0122] The experiment of Example 2 was repeated, and the stoichiometry of the reagents in each cycle and the yield and purity of the product are shown in Table 3 below. After the 8th cycle, carbon tetrachloride is recovered from the previous mother liquor, and the residue is subjected to vacuum distillation. For this, the residue is first thawed and flushed with nitrogen. Thereafter, the temperature was raised while vacuum was used to distill p-nitrotoluene under reduced pressure [temperature: 140°C (initial)-155°C (final); pressure: 40 mm (initial)-10 mm (final) Hg] . The recovered amount of p-nitrotoluene was 0.514 kg (3.75 mol).
[0123] Note: A small amount of p-nitrobenzyl bromide was also found to distill off. The amount of p-nitrotoluene recovered is subtracted from the total amount of p-nitrotoluene fed for calculating the conversion of p-nitrotoluene. Calculated from the data shown in Table 3 below, the yield of p-nitrobenzyl bromide relative to the p-nitrotoluene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com