Method for synthesizing p-nitrobenzoic acid
A technology of p-nitrobenzoic acid and p-nitrotoluene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low production cost, high production cost, unfavorable industrial production, etc. The effect of simple operation, reduced production cost and reduced production risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
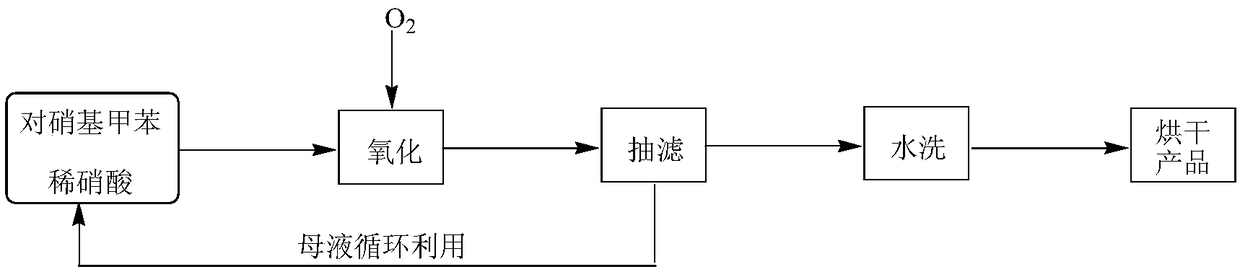
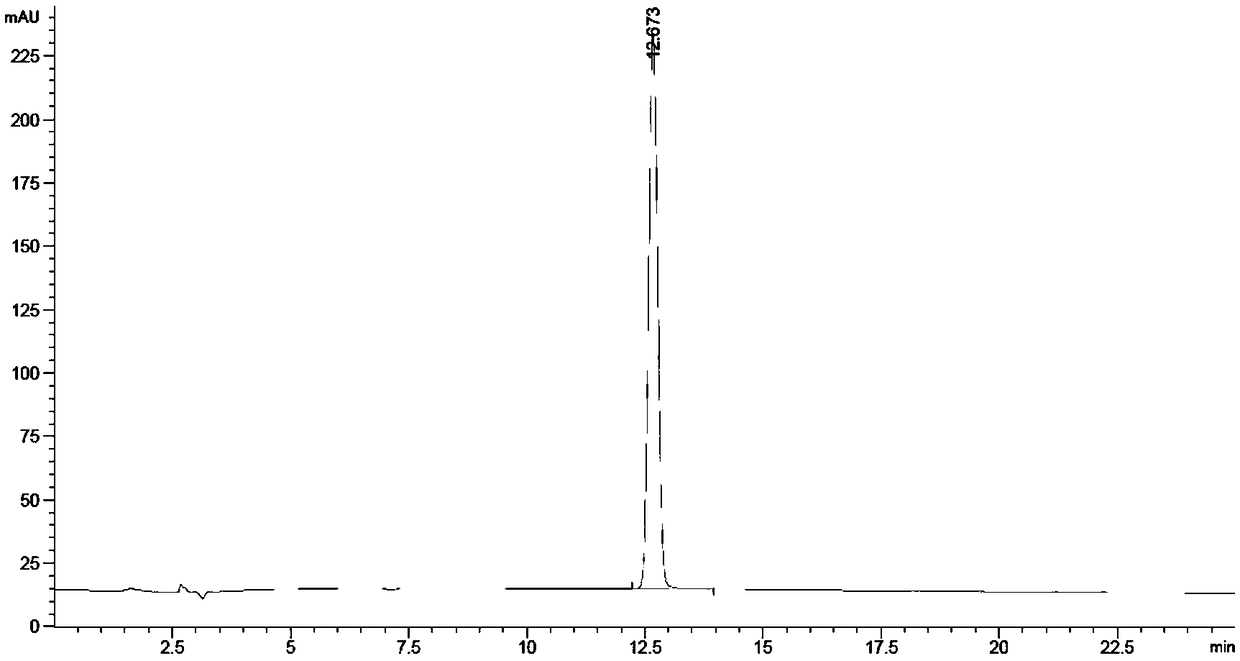
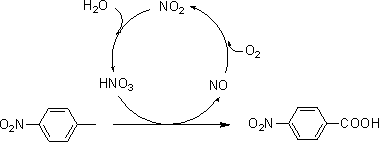
[0026] Put p-nitrotoluene (32.00 g, 233.34 mmol) and dilute nitric acid solution containing 20% nitric acid (containing 536.68 mmol of nitric acid) in a 500 mL autoclave, replace the air in the autoclave with 0.5 MPa oxygen for 4 times, and fill it with oxygen When the pressure reaches 1.0 MPa, start stirring, raise the temperature to 170 °C, stir and react for 1.5 h, stop heating (when the pressure reaches or falls below 0.8 MPa during the reaction, add oxygen to 1.0 MPa in time), cool, filter with suction, and collect the mother liquor for recycling , the filter cake was rinsed with water to neutrality, dried to obtain 36.89 g of light yellow solid, and the purity of the product was 99.88% (high performance liquid chromatography, liquid method Agilent1260 LC liquid chromatograph, area normalization method, chromatographic column Z0RBAX SB-C18, 5 μm, 4.6×250 mm, elution condition: V (methanol): V (water containing 0.8% acetic acid) = 30:70 (0min) → 60:40 (20min) → 60:40 ( 2...
Embodiment 2
[0028] Put p-nitrotoluene (32.00 g, 233.34 mmol) and dilute nitric acid solution containing 10% nitric acid (containing 233.34 mmol of nitric acid) in a 500 mL autoclave, replace the air in the autoclave with 0.6 MPa oxygen for 4 times, and fill it with oxygen When the pressure reaches 2.0 MPa, start stirring, raise the temperature to 230 °C, stir and react for 14.0 h, stop heating (when the pressure is lower than 2.0 MPa during the reaction, add oxygen to 2.0 MPa in time), cool, filter with suction, collect the mother liquor for recycling, filter The cake was rinsed with water until neutral, and dried to obtain 37.42 g of a light yellow solid. The purity of the product was 99.79%, the yield of the product was 95.75%, and the volume of the mother liquor was 148.1 mL.
Embodiment 3
[0030] Put p-nitrotoluene (32.00 g, 233.34 mmol) and dilute nitric acid solution containing 40% nitric acid (containing 933.36 mmol of nitric acid) in a 500 mL autoclave, replace the air in the autoclave with 0.4 MPa oxygen 4 times, and fill it with oxygen When the pressure reaches 0.4 MPa, start stirring, raise the temperature to 120 °C, stir and react for 1.0 h, stop heating (when the pressure is lower than 0.4 MPa during the reaction, add oxygen to 0.4 MPa in time), cool, filter with suction, collect the mother liquor for recycling, filter The cake was rinsed with water until neutral, and dried to obtain 36.11 g of a light yellow solid. The purity of the product was 99.99%, the yield of the product was 92.58%, and the volume of the mother liquor was 135.3 mL.
[0031] The following is the implementation of the mother liquor
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com