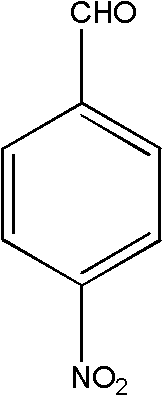
High selectivity synthesis method of p-nitrobenzaldehyde
A high-selectivity p-nitrobenzaldehyde technology, applied in the field of preparation of known compounds, can solve the problems of expensive catalysts, difficult recycling and recycling, etc., to reduce the generation of organic by-products, shorten the reaction time, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
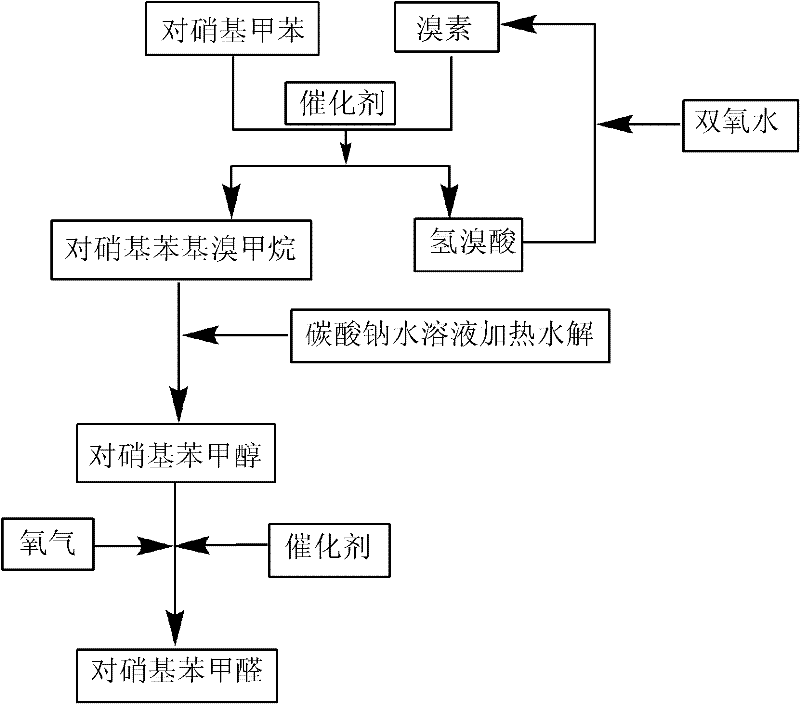
[0027] In a 250mL three-necked flask, add 100g of dichloroethane, 20.5g of p-nitrotoluene, 1.0g of di(2-ethyl)hexyl peroxydicarbonate dissolved in 16g of dichloroethane, at 45°C, Slowly add 12.3 g of bromine dropwise in 30-60 minutes. After the dropwise addition, maintain the temperature and stir for 3 hours to form a light yellow solution. After adding 0.5 g of di(2-ethyl)hexyl peroxydicarbonate dissolved in 8.0 g of dichloroethane, slowly add 9.5 g of 27% hydrogen peroxide dropwise in 30 minutes, and control the temperature at 65-68° C. to stir the solution for 4 hours. Add 65g of 30% sodium carbonate aqueous solution to the bromination solution, stir and raise the temperature to recover 80g of dichloroethane, and stir the mixed solution at 80-95°C for 16h. Stand to separate the aqueous phase and the organic phase containing p-nitrobenzyl alcohol. Transfer the organic phase into a stainless steel pressure vessel, 0.23g triphenylphosphine bismuth salt, at a temperature of 50...
Embodiment 2
[0029] In a 250mL three-necked flask, add 80g of dichloroethane, and then add 20.5g of p-nitrotoluene, 1.2g of di(2-ethyl)hexyl peroxydicarbonate dissolved in 19g of dichloroethane, at 45 At ℃, slowly add 12.7g of bromine dropwise within 30min. After the dropwise addition, stir and react at 50°C for 3.5h, then slowly add 9.5g of 27% hydrogen peroxide dropwise within 30min, and control the temperature at 60-62°C to stir the solution for 5h. Add 65g of 30% sodium carbonate aqueous solution to this bromination solution, stir and raise the temperature to recover 70g of dichloroethane, and stir the mixed solution at 80-95°C for 16h. Stand to separate the aqueous phase and the organic phase containing p-nitrobenzyl alcohol. Transfer the organic phase into a stainless steel pressure vessel, 0.23g triphenylphosphine bismuth salt, at a temperature of 50-70°C, 8.1×10 5The reaction was stirred under Pa oxygen atmosphere pressure for 28h. After the reaction, add 20 g of water under norm...
Embodiment 3
[0031] In a 250mL three-necked flask, add 80g of dichloroethane, followed by adding 20.5g of p-nitrotoluene, 1.2g of di(2-ethyl)hexyl peroxydicarbonate dissolved in 19g of dichloroethane, at 45°C Slowly add 12.7g of bromine dropwise within 30min. After the dropwise addition, stir and react for 3.5h, slowly add 9.5g of 27% hydrogen peroxide dropwise within 30min, control the temperature at 65-68°C and stir the solution for 5h. Add 65g of 30% sodium carbonate aqueous solution to this bromination solution, stir and raise the temperature to recover 80g of dichloroethane, and stir the mixed solution at 80-95°C for 16h. The aqueous phase and the organic phase containing p-nitrobenzyl alcohol and the like were separated by standing. Transfer the organic phase into a stainless steel pressure vessel, 0.23g triphenylphosphine palladium salt, at a temperature of 50-70°C, 8.1×10 5 The reaction was stirred for 22 h under Pa oxygen atmosphere pressure. After the reaction, add 20 g of wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com