Method for synthesizing TNPP and TAPP at high yield and high efficiency
A high-efficiency and high-yield technology, applied in organic chemistry and other directions, can solve the problems of difficult purification, difficult product separation, affecting product purity, etc., and achieve the effect of improving purity and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1, TNPP
[0033] Weigh 3.5g (23.23mmol) of p-nitrobenzaldehyde and 4.0ml (42.33mmol) of propionic anhydride, add them to 150ml of propionic acid under stirring, and heat the solution to 135°C;
[0034] Take 1.7ml (23.23mmol) freshly steamed pyrrole, dissolve it in 5ml propionic acid, then slowly add it dropwise to the above solution, stir and reflux for 40min to obtain a tar-like substance; cool, place for 24h, filter to obtain a black solid, wash with distilled water, and dry , the powder solid is TNPP crude product;
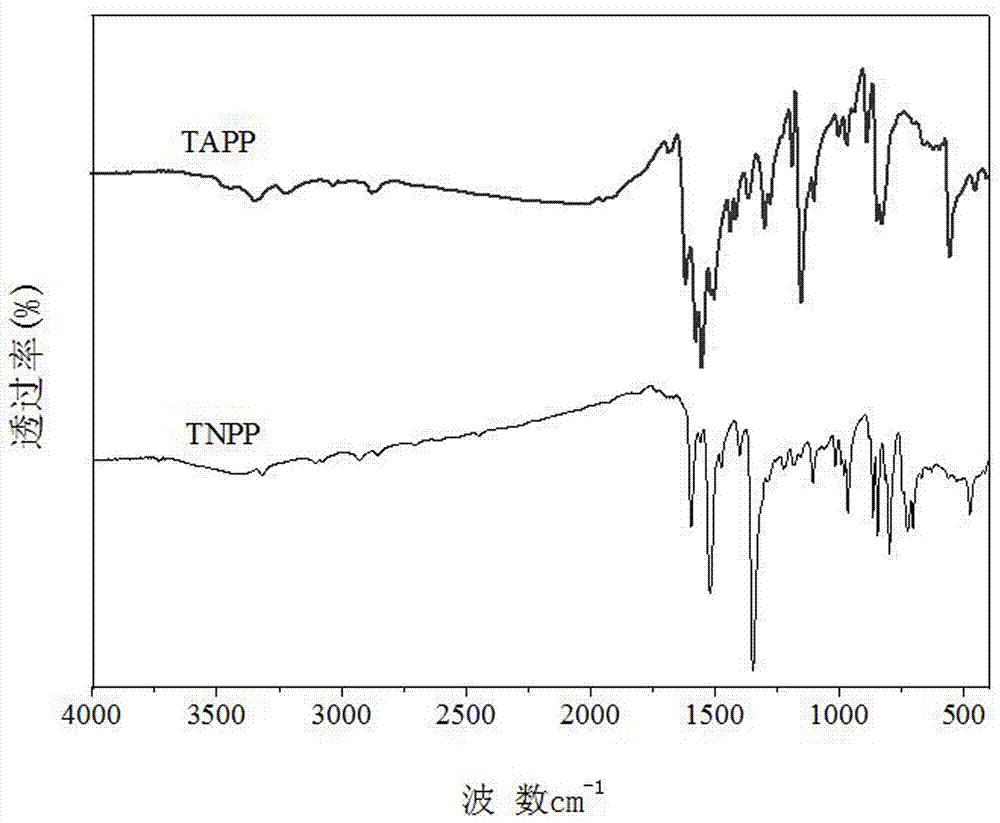
[0035] Add crude TNPP to 30ml of pyridine, heat to 110°C, reflux and stir for 1h, cool to room temperature, place in refrigerator (-4°C) for 10h, filter the tarry mixture, and wash the solid matter repeatedly with acetone and distilled water until no Then it is black, and 1.2-1.4 g of TNPP product is obtained, the purity is 86%, and the yield is 27-30%.
[0036] The elemental analysis of TNPP products is shown in Table 1; th...
Embodiment 2
[0037] The synthesis of embodiment 2, TAPP
[0038] Weigh 1.0 g of TNPP synthesized in Example 1, dissolve it in 50 ml of concentrated hydrochloric acid solution (concentration is 12 mol / l), react at room temperature for 1 h under nitrogen bubbling, and obtain TNPP solution;
[0039] Weigh 4.5g SnCl 2 ·H 2 O, dissolved in 60ml~70ml concentrated hydrochloric acid solution, added dropwise to the above TNPP solution under the protection of nitrogen, heated in a water bath to 75~80°C, and reacted for 30min; then use a cold water bath instead of a hot water bath to cool down to below 20°C;
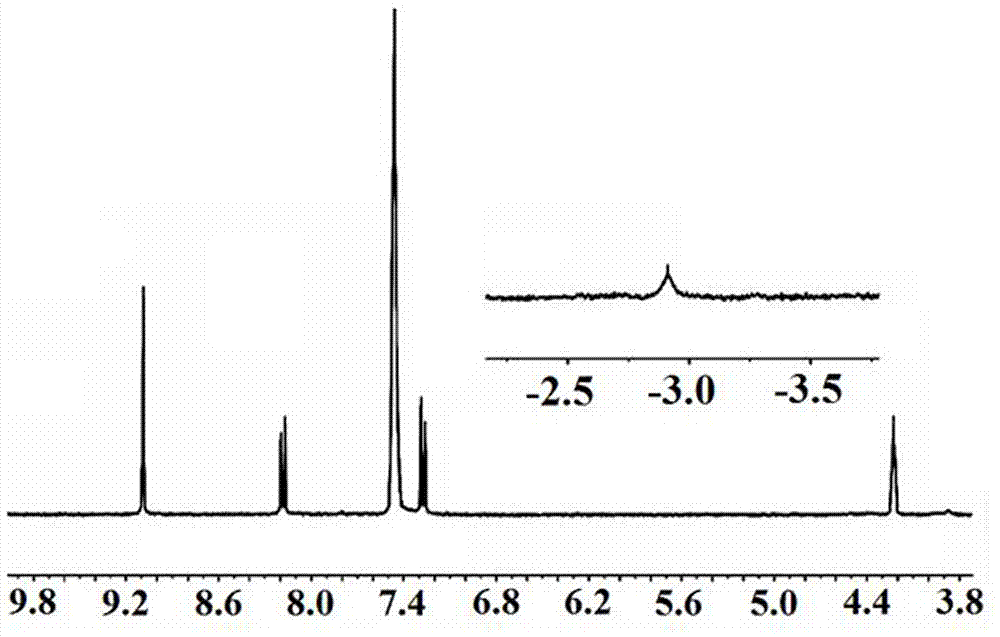
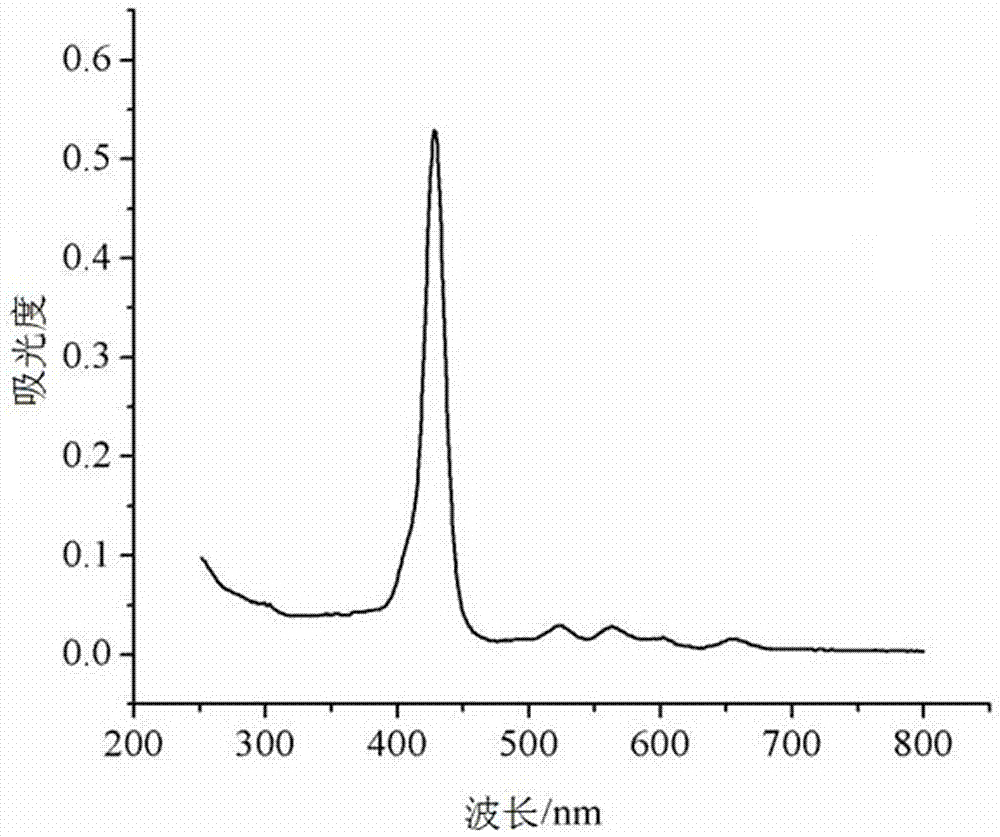
[0040]Slowly add concentrated ammonia water (concentration 22% to 25%) into the above solution dropwise until pH = 8 to 9, then extract with chloroform for several times by Soxhlet extraction until the filtrate no longer turns black, collect the organic phase, and rotary evaporate The organic phase was dried, and finally dried under vacuum at 50° C. for 3 hours to obtain 0.7 g of purple solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com