Method for determining 4-nitrobenzaldehyde in chloramphenicol or preparations thereof through derivatization HPLC-UV/Vis
A technology of HPLC-UV and nitrobenzaldehyde, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inapplicability to laboratory routine testing and lack of specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 1.3. Preparation of solution
[0042] 4-Nitrobenzaldehyde stock solution: Take about 10mg of 4-nitrobenzaldehyde, accurately weigh it, place it in a 10mL volumetric flask, dilute to the mark with acetonitrile-water with a volume concentration of 70% acetonitrile, and shake it well. .
[0043] 2-Nitrophenylhydrazine hydrochloride, 3-nitrophenylhydrazine hydrochloride, 4-nitrophenylhydrazine hydrochloride and 2,4-dinitrophenylhydrazine hydrochloride test solution (hereinafter referred to as 2-NPH ·HCl, 3-NPH·HCl, 4-NPH·HCl and 2,4-DNPH·HCl): Take about 100mg of each reagent, accurately weigh it, and place it in a 10mL volumetric flask. Dilute to the mark with 80% acetonitrile. Shake well. Need to be freshly prepared. Among them, 2,4-dinitrophenylhydrazine hydrochloride is first dissolved with an appropriate amount of N,N-dimethylacetamide.
[0044] 1.4. Experimental conditions for derivatization
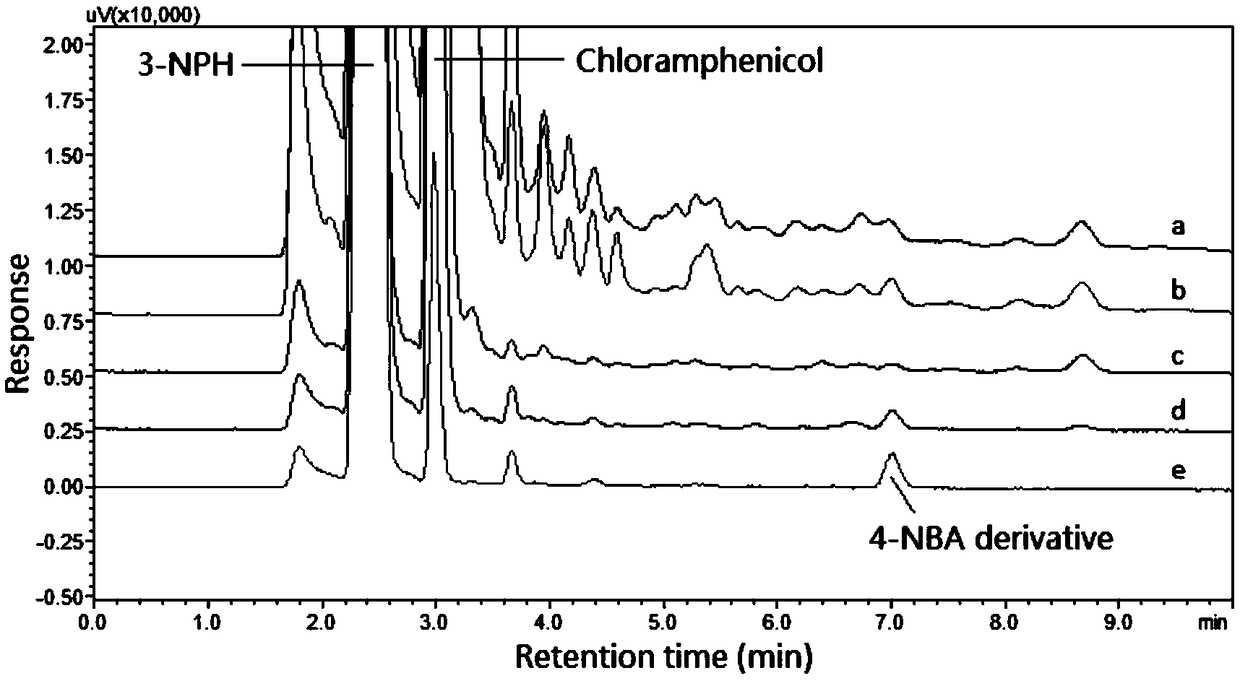
[0045] Precisely weigh the appropriate amount of medicines, or accurately pipet...
Embodiment 1
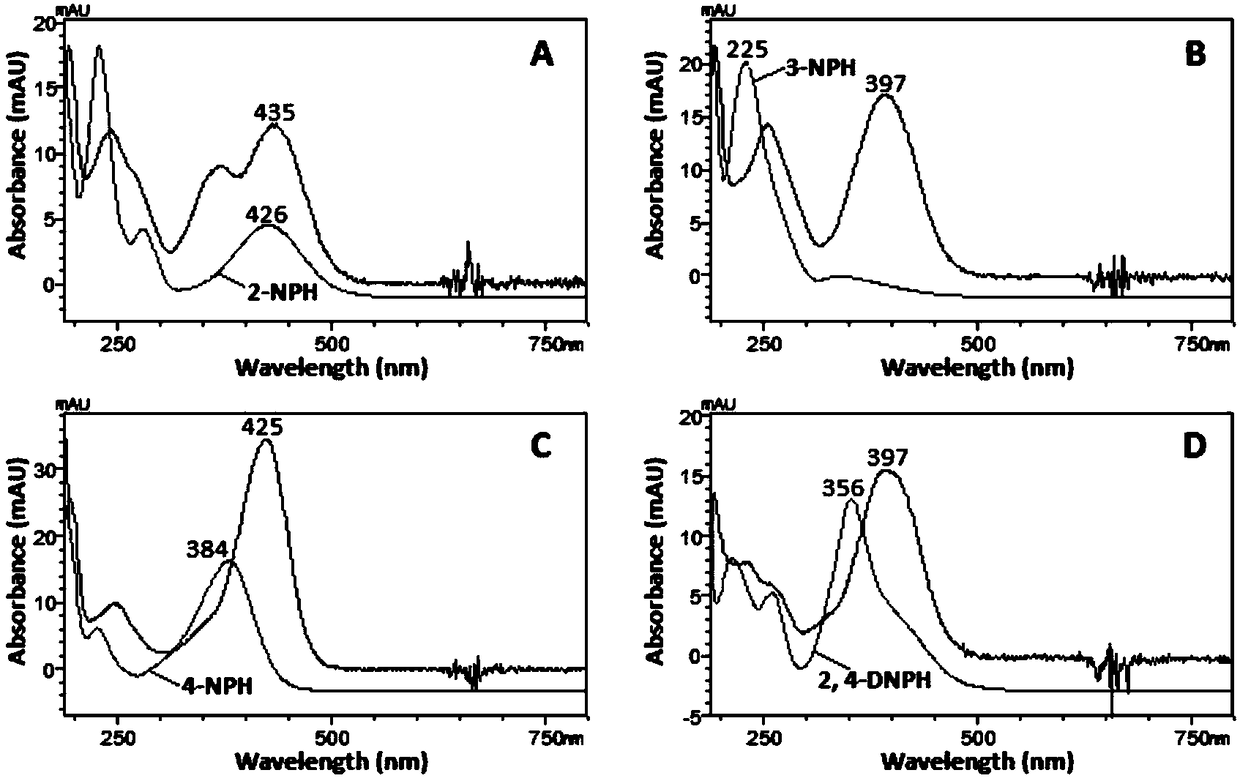
[0049] Taking 4-nitrobenzaldehyde as the test substance, it was derivatized with 4 nitrophenylhydrazine derivatizing reagents of the same concentration. See 1.4 for derivatization experimental conditions. Under the same chromatographic conditions, scan each derivatization product with DAD, record the spectrum and chromatogram of the product, and evaluate the quality of the derivatization reagent with the maximum absorption wavelength and absorption intensity of the product.
[0050] figure 1 It shows that except for 3-nitrophenylhydrazine, the maximum absorption wavelengths of the other three derivatization reagents are all above 350nm, which are 426nm (2-nitrophenylhydrazine), 384nm (4-nitrophenylhydrazine) and 356nm ( 2,4-Dinitrophenylhydrazine), which can be attributed to the electron withdrawing ability of the nitro group at the ortho and / or para position on the benzene ring of phenylhydrazine. The maximum absorption wavelength of 3-nitrophenylhydrazine is only 225nm, and th...
Embodiment 2
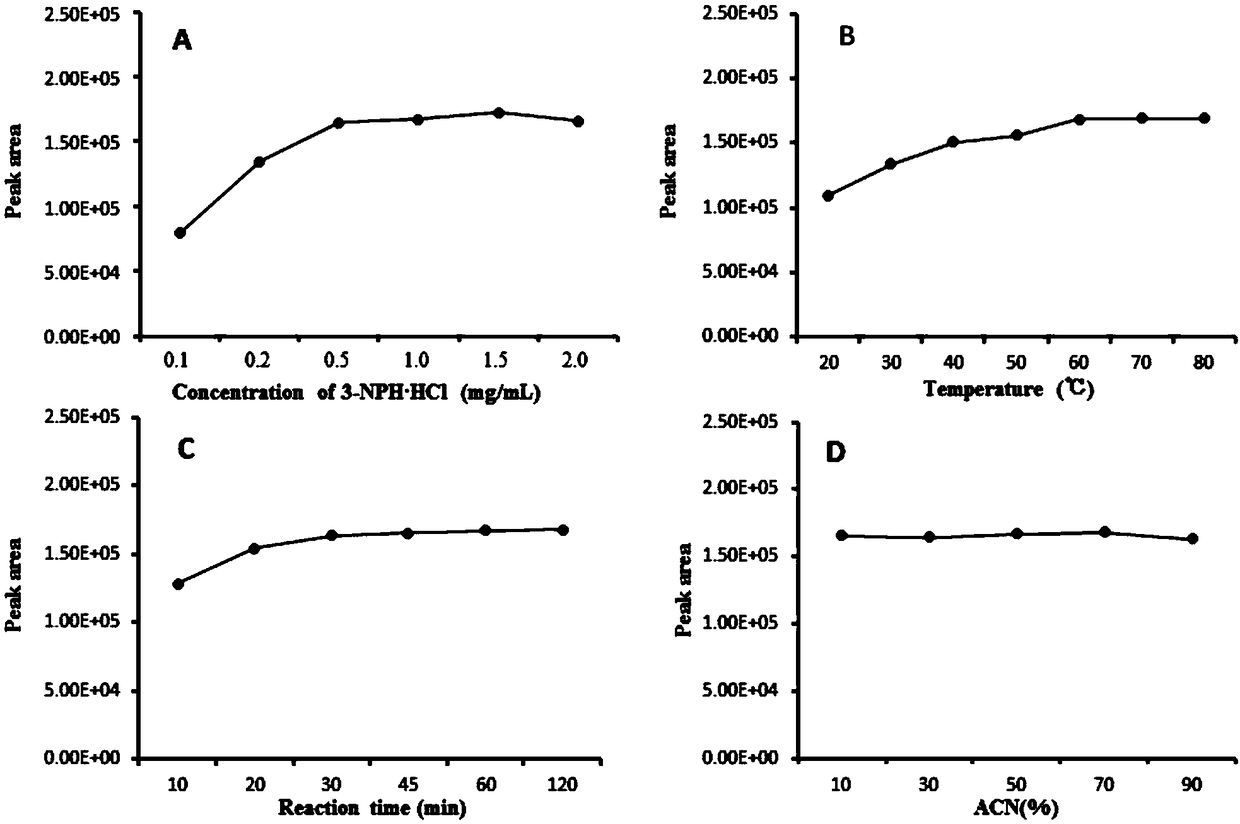
[0054] In order to ensure that the derivatization reaction proceeds quickly and the reaction is complete, the amount of derivatization reagent in the system, the reaction time, the reaction temperature and the ratio of the organic phase need to be investigated. Taking the peak area of the resulting 4-nitrobenzaldehyde derivatization product as an indicator, a single factor test investigated different concentrations of 3-nitrophenylhydrazine hydrochloride (0.1, 0.2, 0.5, 1.0, 1.5, 2.0 mg / mL ), different reaction temperatures (20, 30, 50, 60, 70, 80℃), different reaction times (10, 20, 30, 45, 60, 120min) and the organic ratio of the reaction system (10%, 30 %, 50%, 70%, 90%) on the efficiency of the derivatization reaction.
[0055] by image 3 A. It can be seen that the peak area of the derivatization product increases with the increase of the concentration of the derivatization reagent, until the concentration of 3-nitrophenylhydrazine hydrochloride is 500μg / mL (equivalent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com