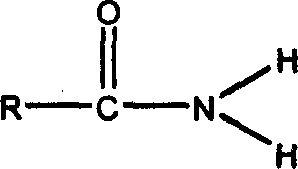
Acidamide derivative preparation method
A technology of amide derivatives and catalysts, applied in the field of amide derivatives, can solve the problems of easy generation of waste acid or waste alkali, strong corrosion of catalyst strong acid or strong alkali, difficulty in separation and reuse, etc., to avoid corrosion and facilitate operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0006] The preparation of embodiment 1.N-(1-hydroxyl-2,2,2-trichloroethyl)acrylamide
[0007] Experimental procedure: (1) in 1000ml there-necked flask, add the acrylamide of 71.1 grams (1mol), 0.5 gram stopper phenothiazine, 15g Amberlyst35 (the strong acid cation of the H type that Shanghai Rohm and Haas Chemical Co., Ltd. produces Exchange resin) and 150 milliliters of methanol, nitrogen, after a few minutes, put the reaction bottle into 80-90 ℃ oil bath, then slowly drop into 100 milliliters of methanol solution of 181.5 grams of chloral hydrate under stirring, about 2 to 3 After the addition was completed within 1 hour, the reaction was continued with heating and stirring for 1 to 2 hours under the protection of nitrogen.
[0008] (II) Filter the reaction mixture, wash the H-type cation exchange resin several times with methanol, and combine the washing solution and the mother liquor.
[0009] (III) The obtained liquid was evaporated to dryness under reduced pressure at 5...
Embodiment 2
[0011] The preparation of embodiment 2.N-(1-hydroxyl-2,2,2-trichloroethyl)methacrylamide
[0012] Experimental procedure: (1) In a 1000ml three-necked flask, add 85.1 grams (1mol) of methacrylamide, 0.5 grams of phenothiazine, 15g of Amberlyst and 150 milliliters of methanol, and place the reaction bottle at 80-90°C after purging nitrogen for several minutes In an oil bath, slowly add 181.5 g of chloral hydrate in 100 ml of methanol solution dropwise under stirring, and the addition is completed in about 2 to 3 hours, and the reaction is continued with heating and stirring for 1 to 2 hours under the protection of nitrogen.
[0013] (II) Filter the reaction mixture, wash the H-type cation exchange resin several times with methanol, and combine the washing solution and the mother liquor.
[0014] (III) The obtained liquid was evaporated to dryness under reduced pressure at 50° C., and the crude product was obtained after drying.
[0015] (IV) Recrystallize the crude product fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com