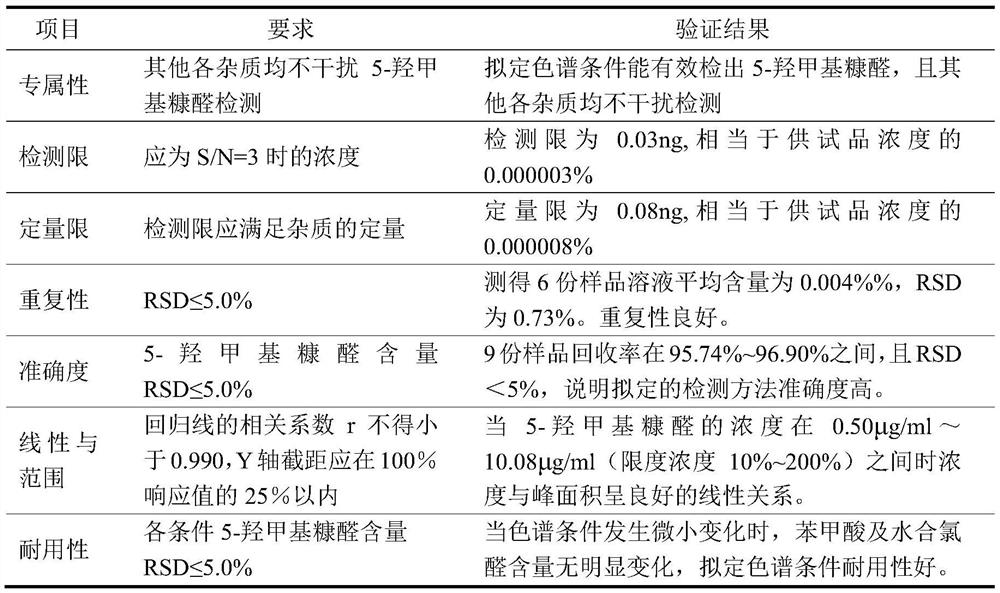
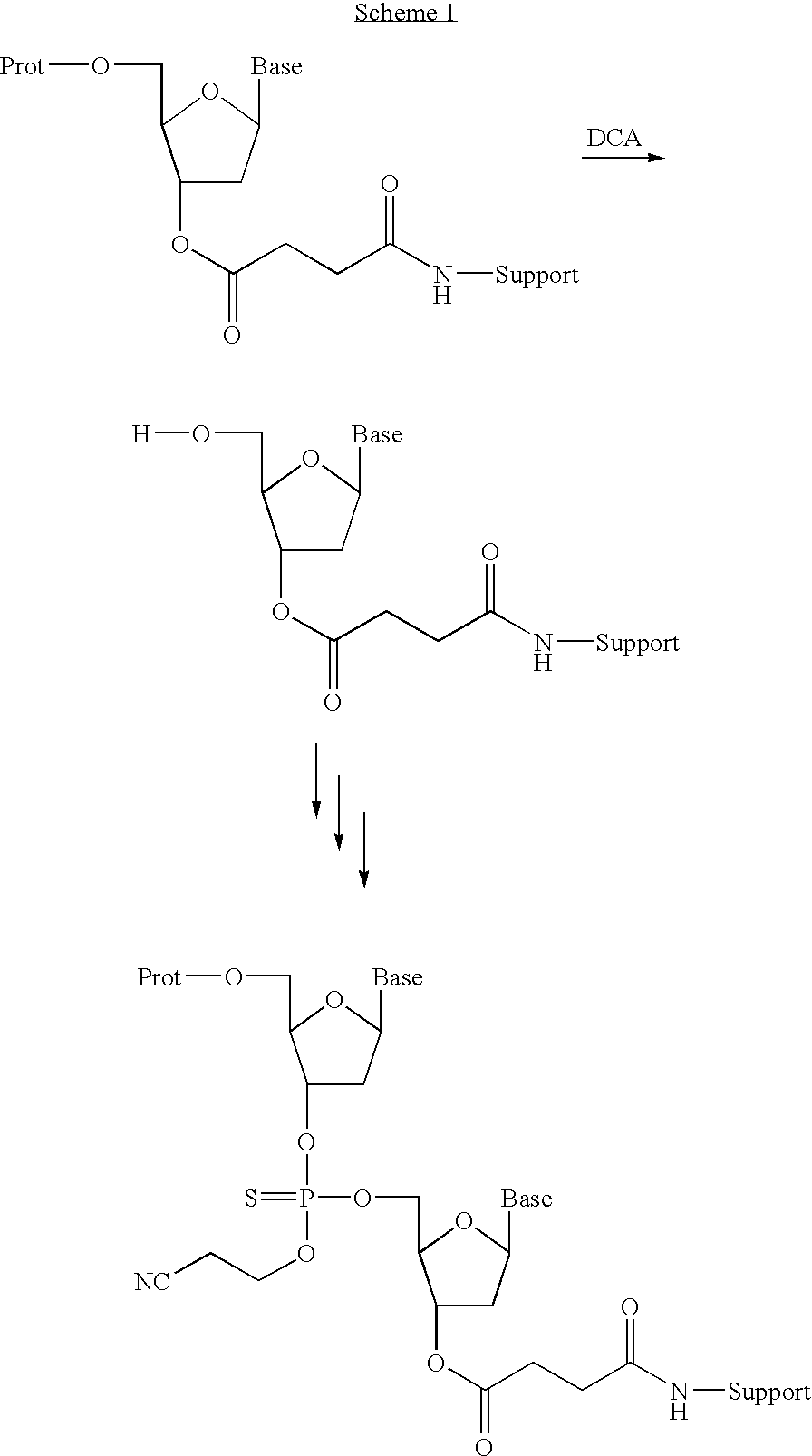
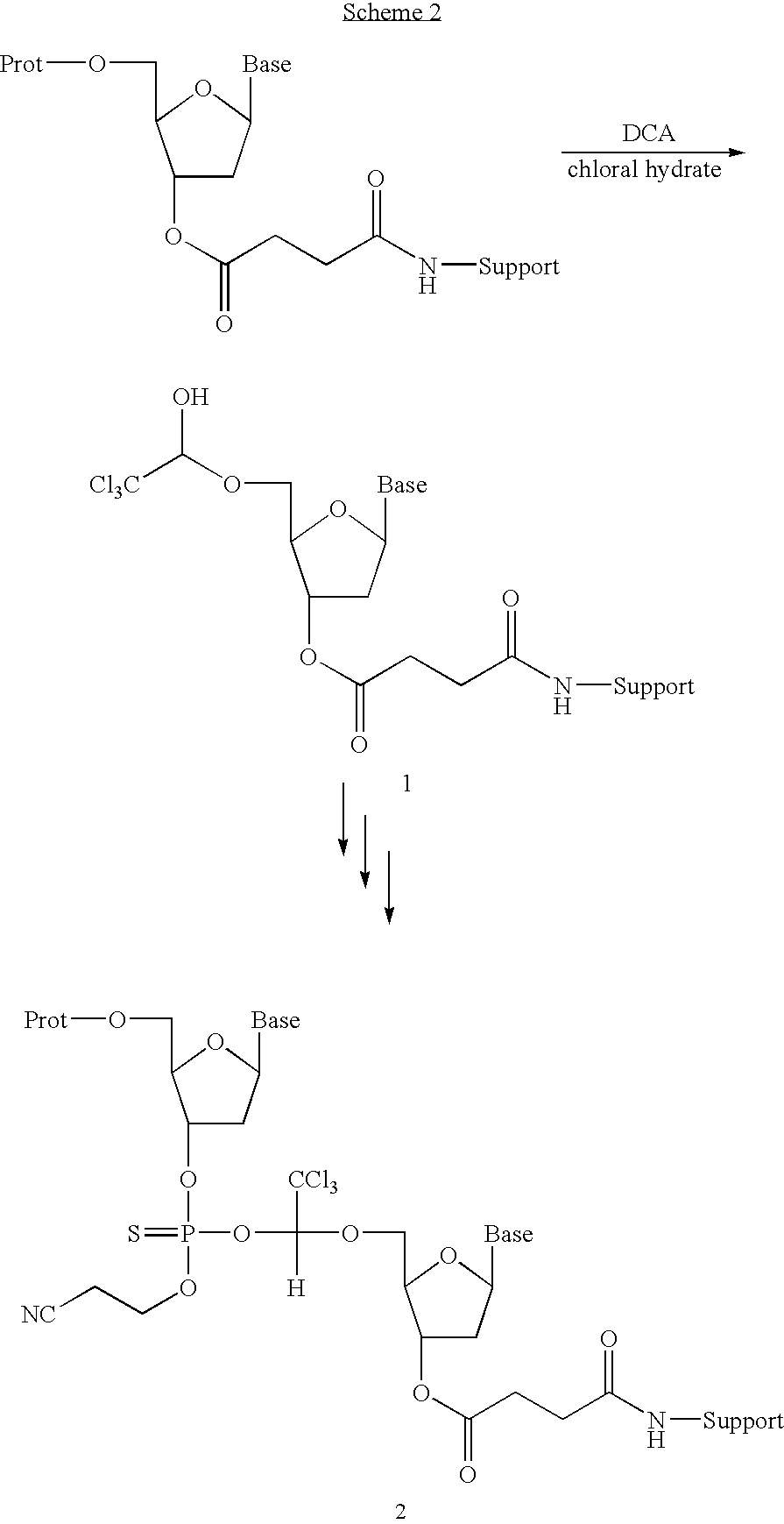
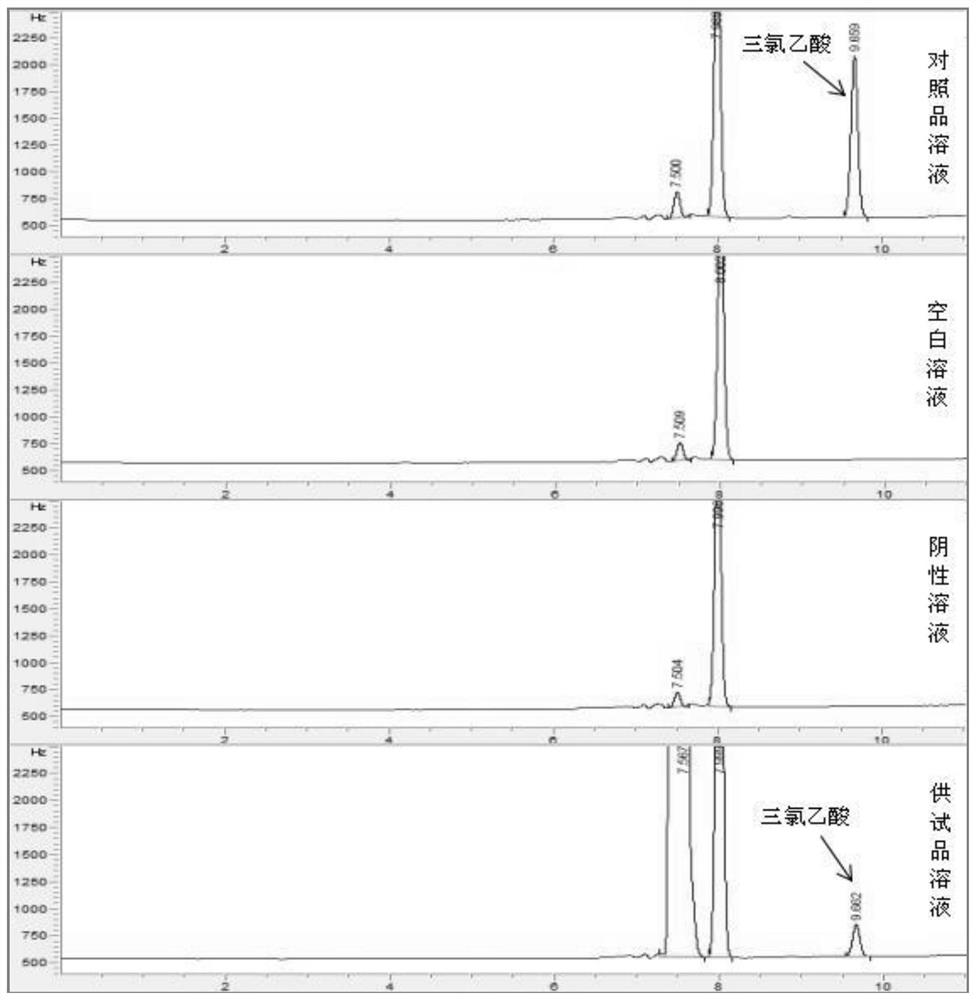
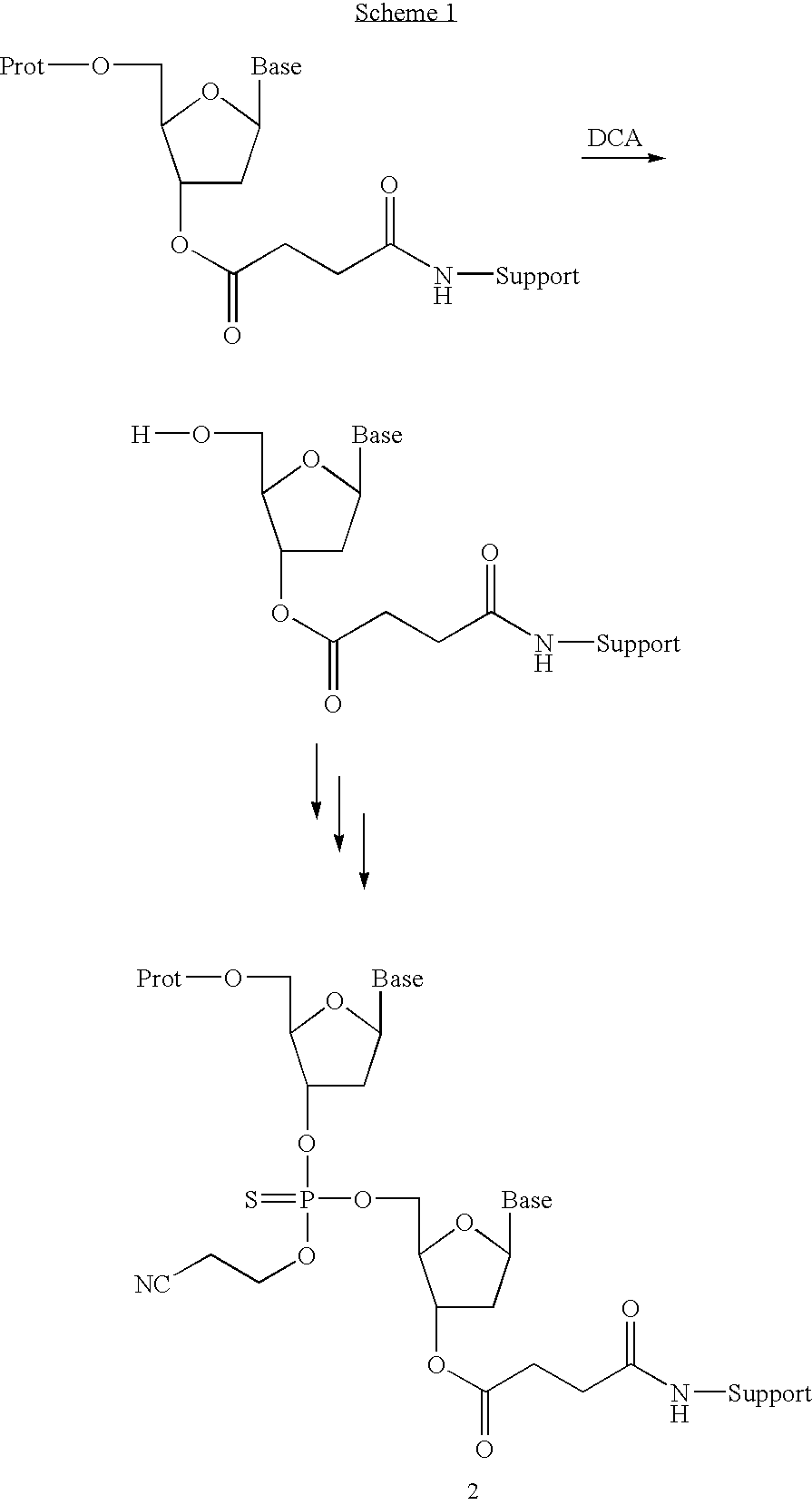
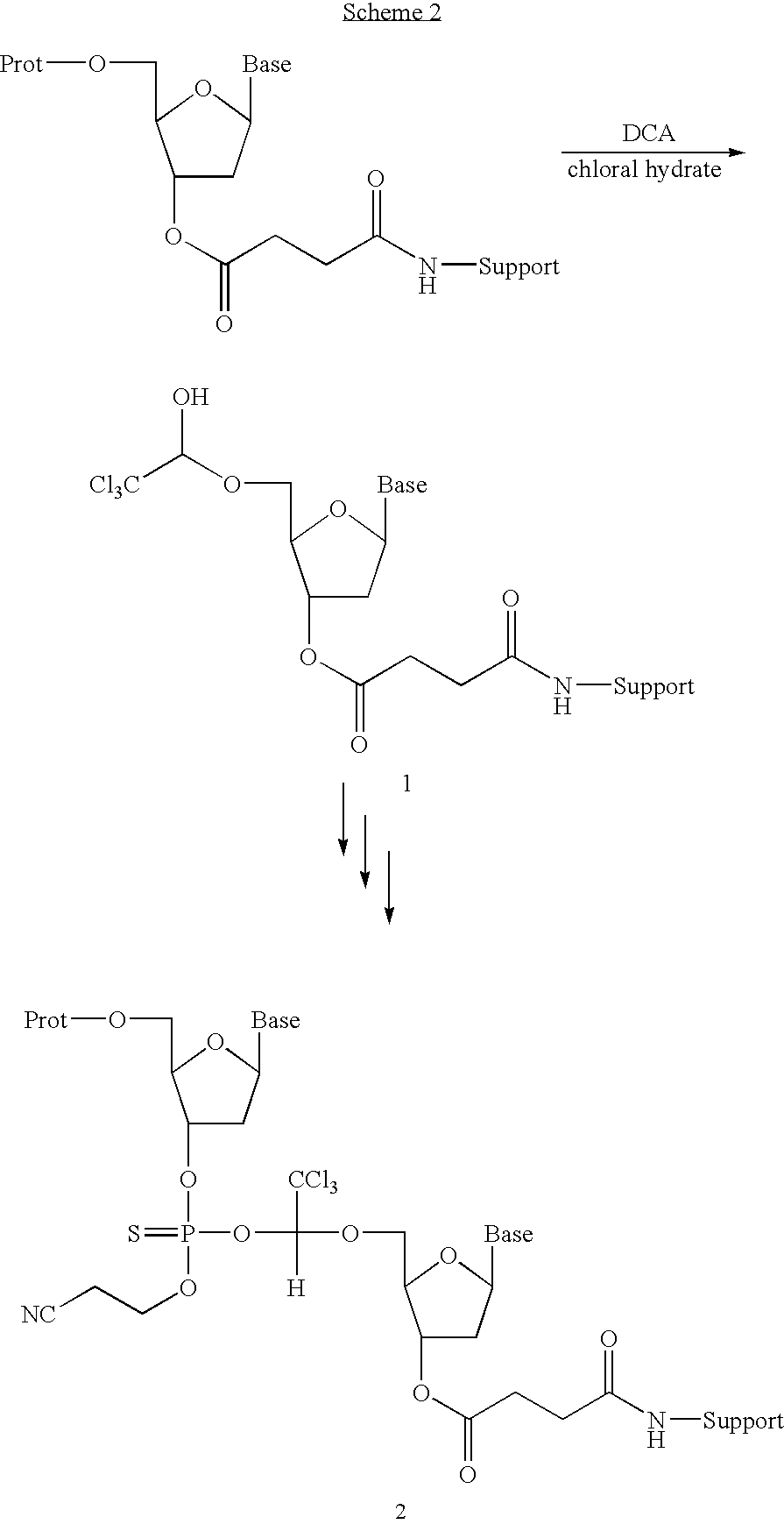
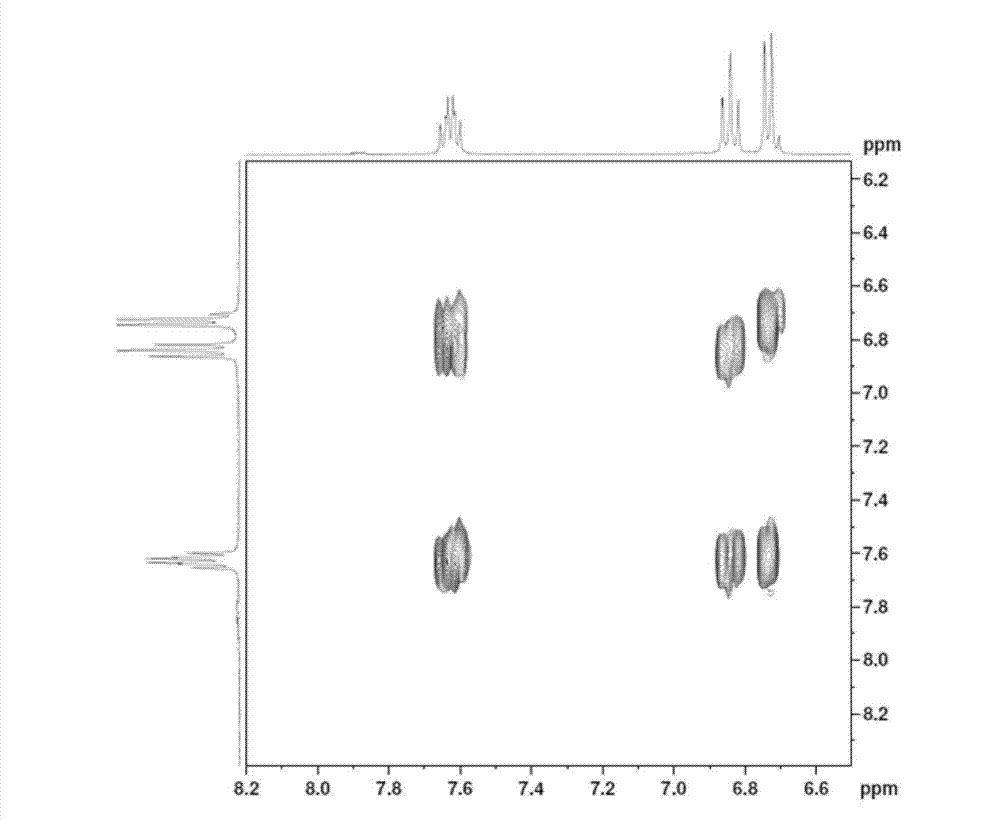
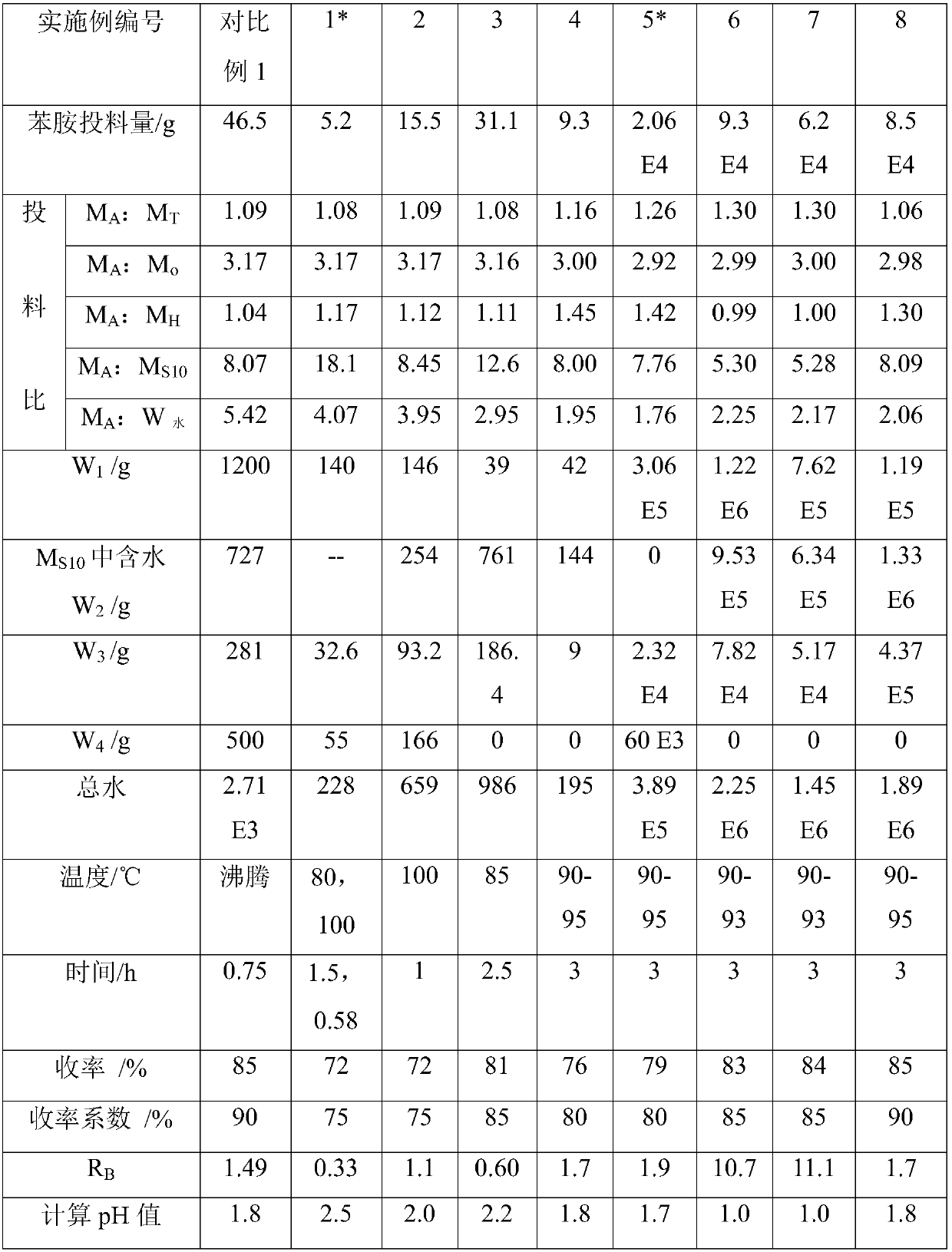
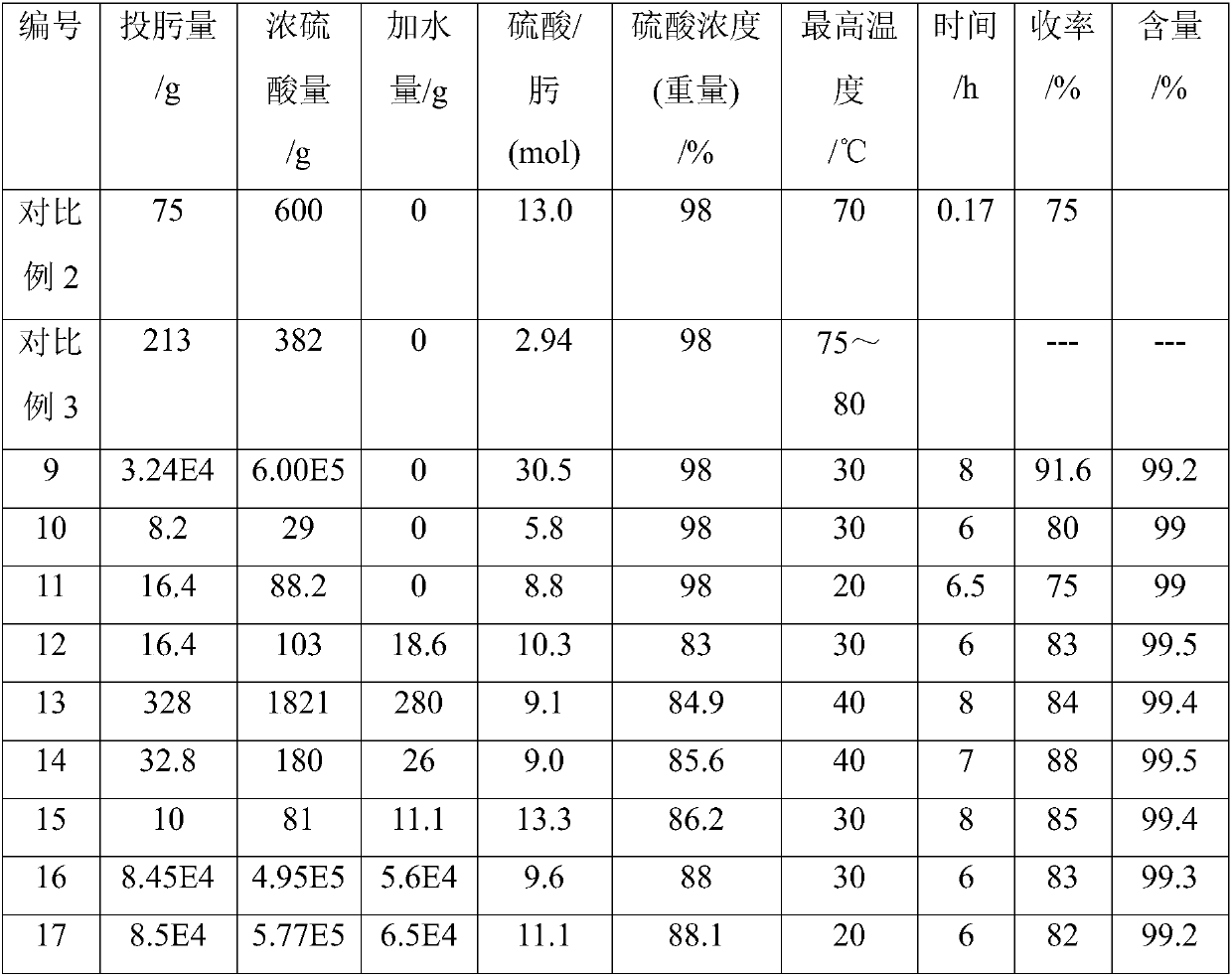
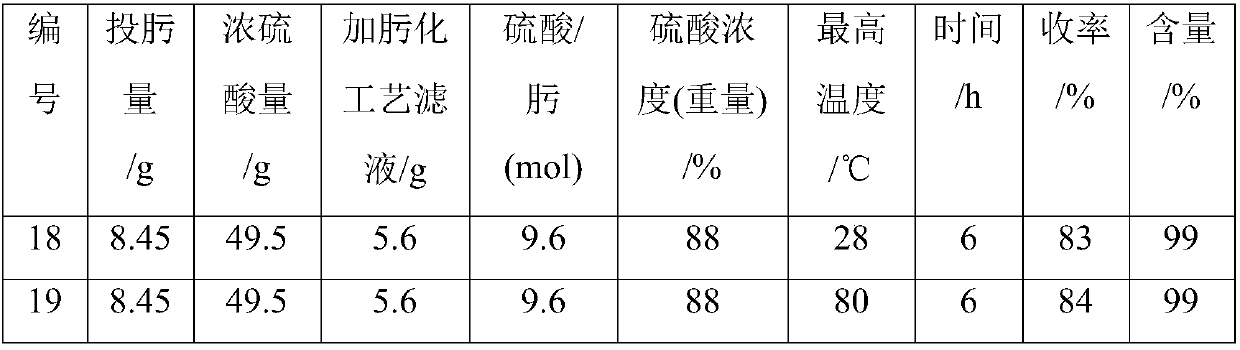
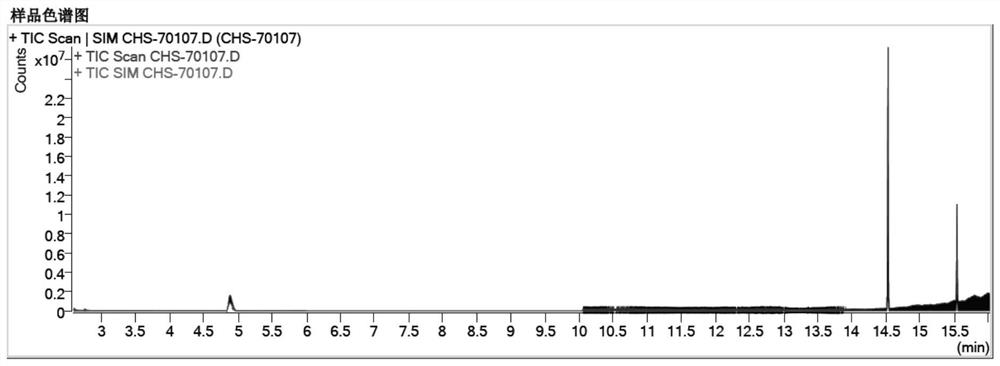
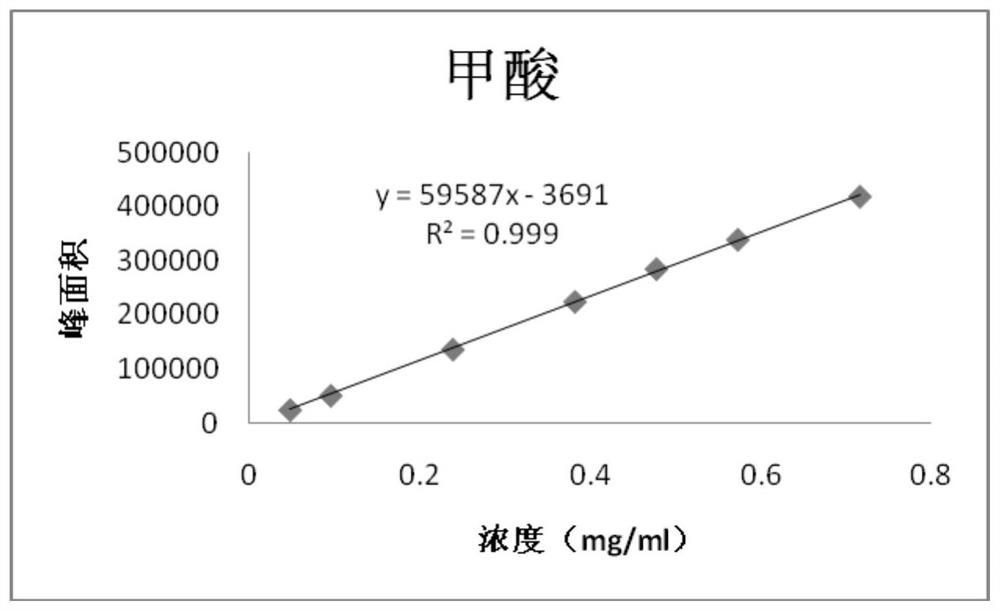
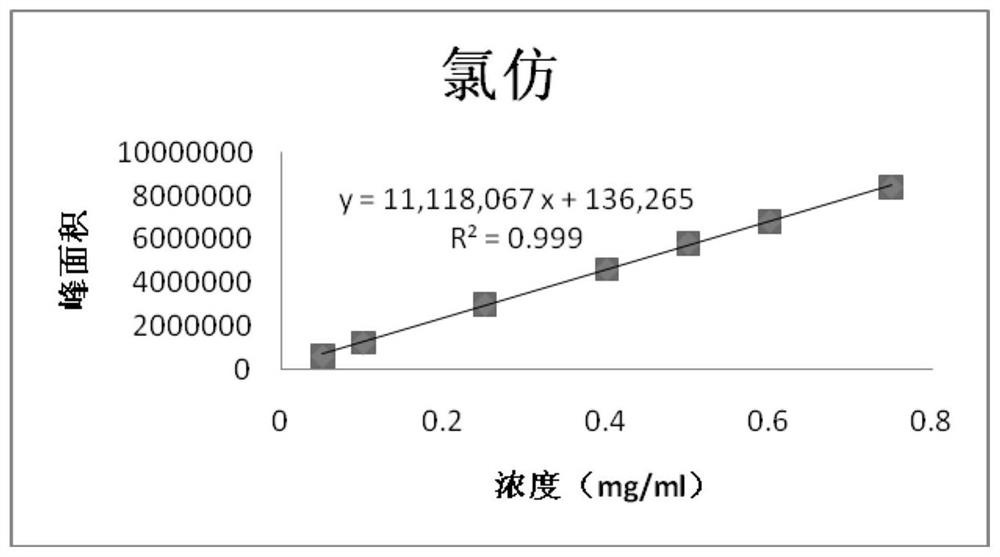
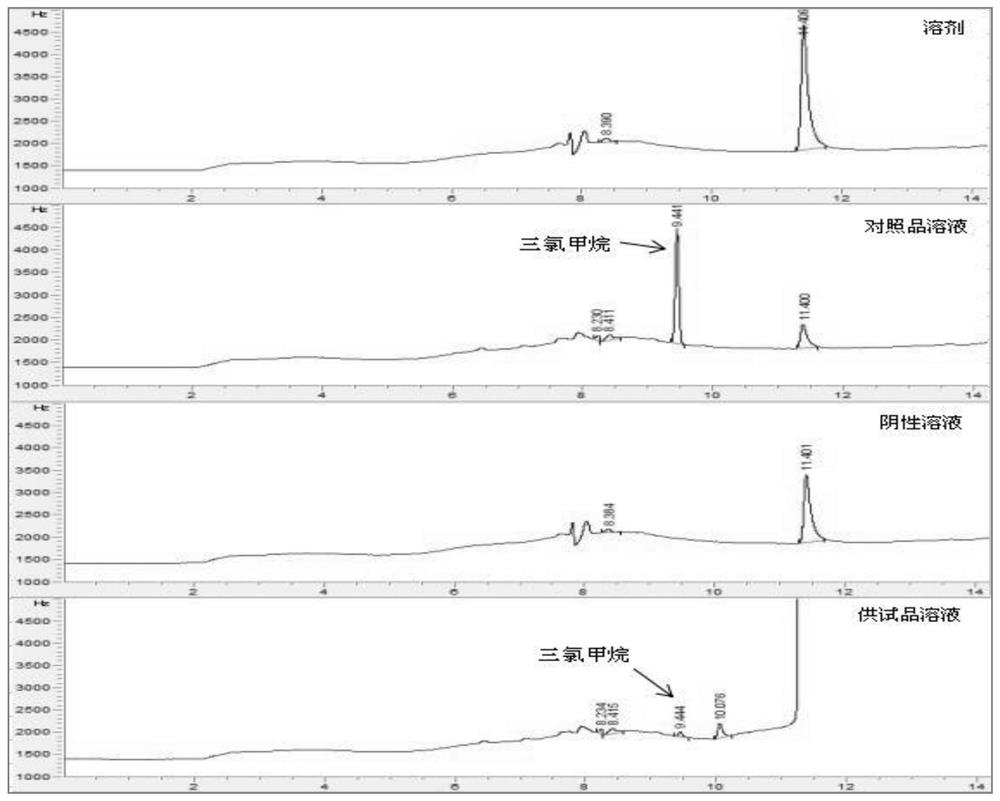
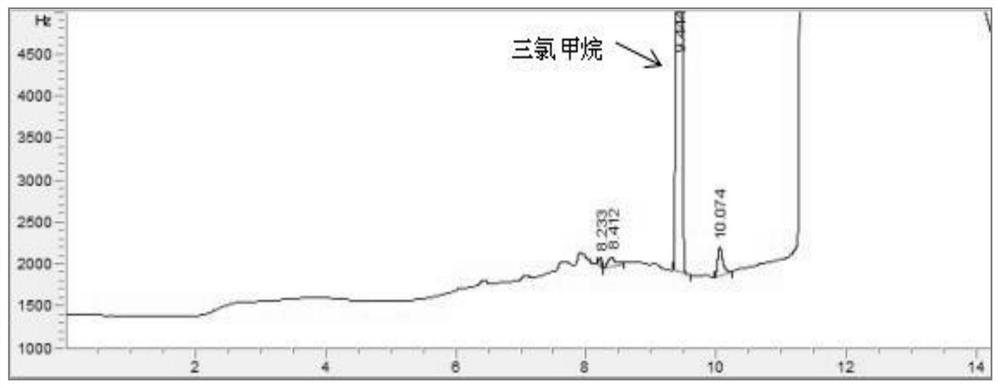
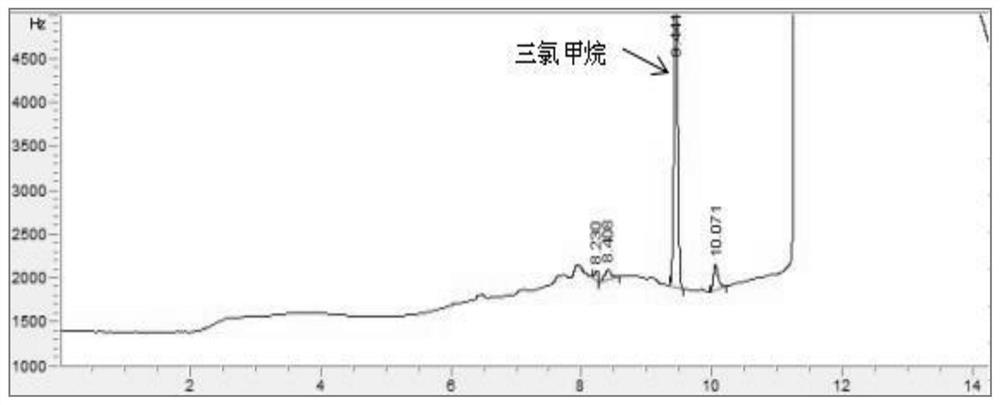
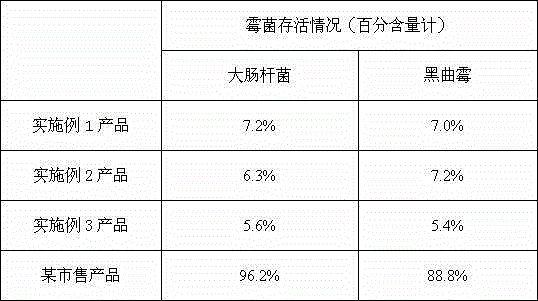
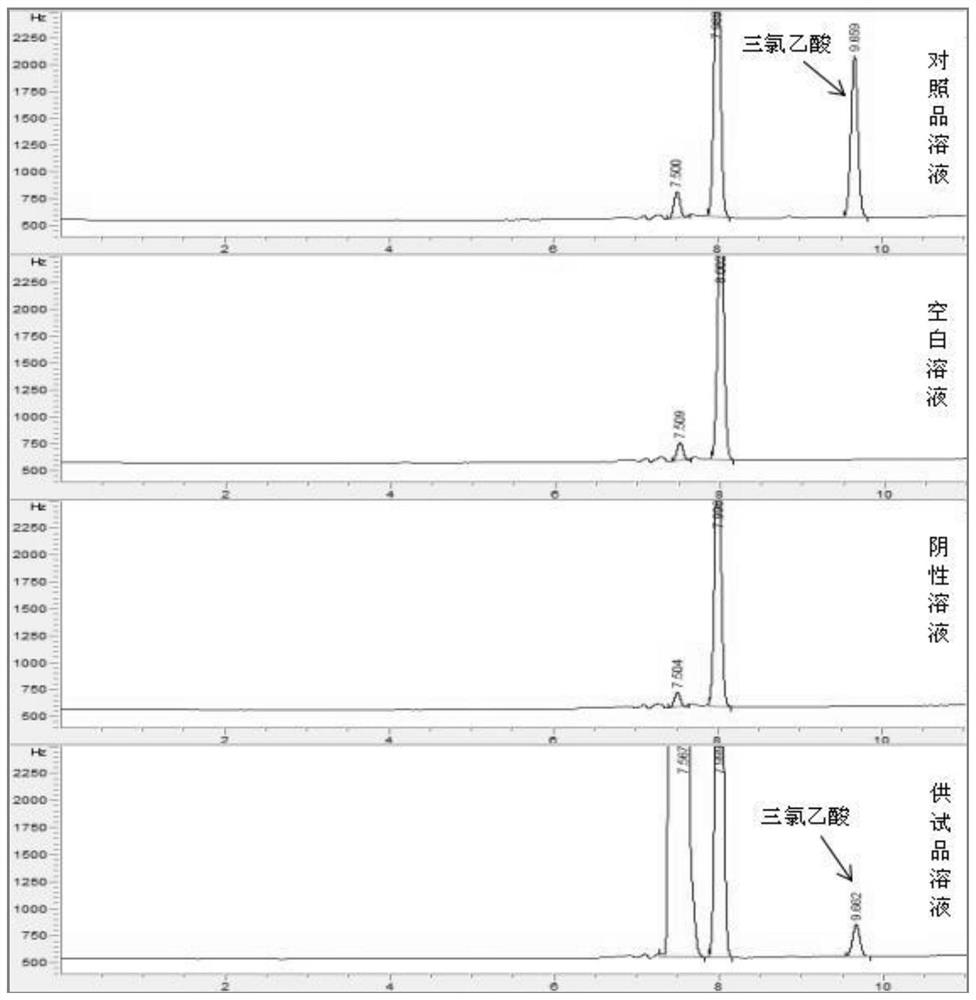
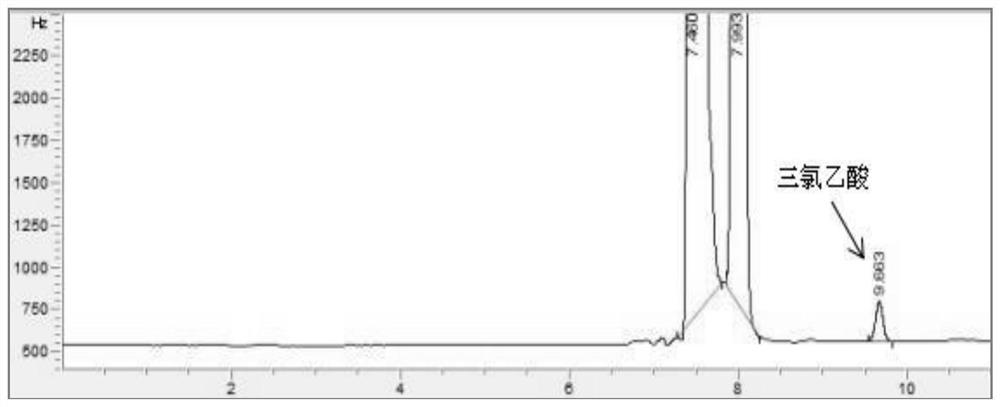
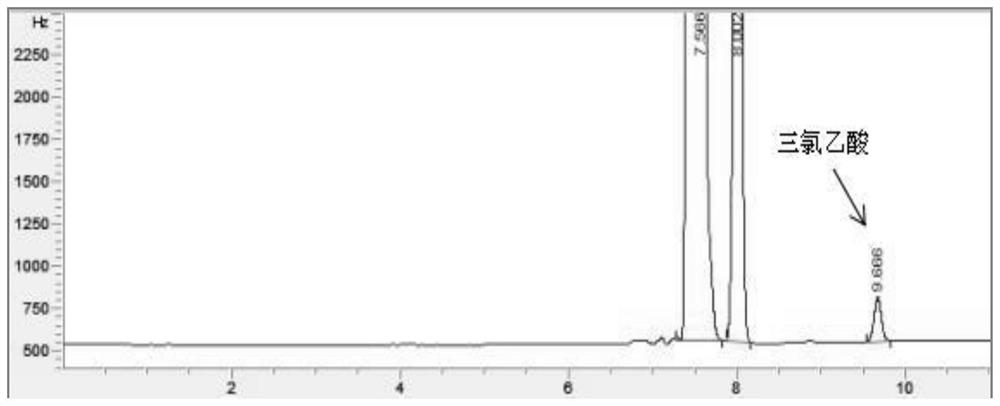
Patents
Literature
62 results about "Chloral hydrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to calm you just before surgery or other procedures.
Itching-relieving hydrogel dressing and preparation method thereof
ActiveCN105288707ALess irritatingNo side effectsHydroxy compound active ingredientsAbsorbent padsAdjuvantTreatment effect
The invention relates to the technical field of medical dressings, in particular to an itching-relieving hydrogel dressing and a preparation method thereof. The itching-relieving hydrogel dressing comprises cortex phellodendri extract, radix scutellariae extract, radix sophorae flavescentis extract, fructus cnidii extract, cortex dictamni extract, mint, glycerol, carbomer, chloral hydrate, epsilon-polylysine, camphor, borneol, propylene glycol and purified water. The itching-relieving hydrogel dressing has the advantages that by being prepared from pure traditional Chinese medicines except for adjuvants, the itching-relieving hydrogel dressing is little in irritation, free from toxic or side effect, safe to use, good in permeability and treatment effect and quick in action and can be used for relieving symptoms such as skin and mucosa itching caused by cicatrices formed after burn, scald, operations and vagina or various other wounds.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Process for synthesis of lonidamine
ActiveCN1594297AReduce pollutionLow costOrganic chemistryAntineoplastic agentsSynthesis methodsCarboxylic acid
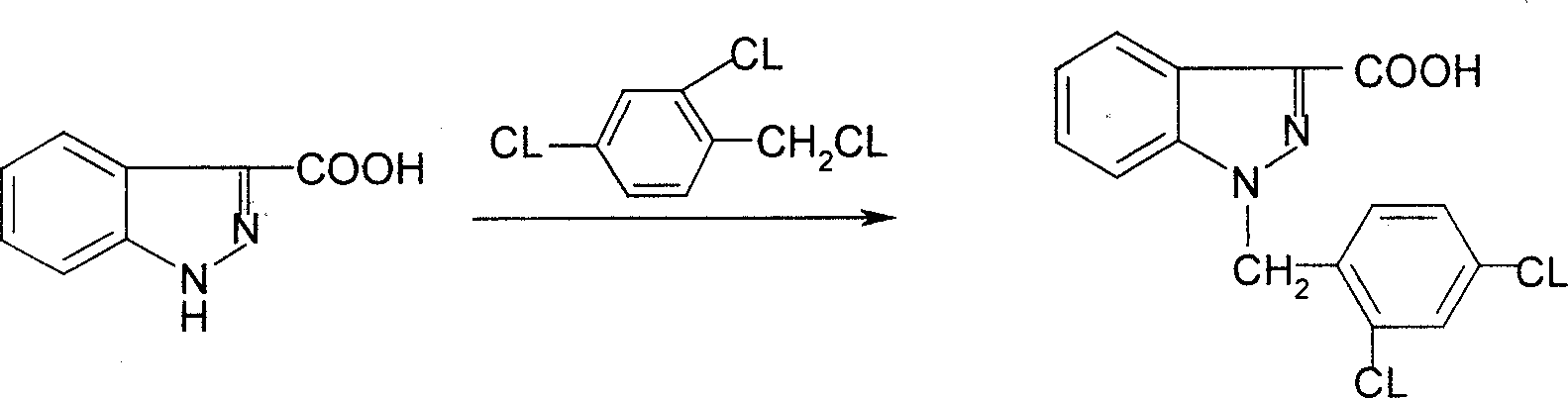
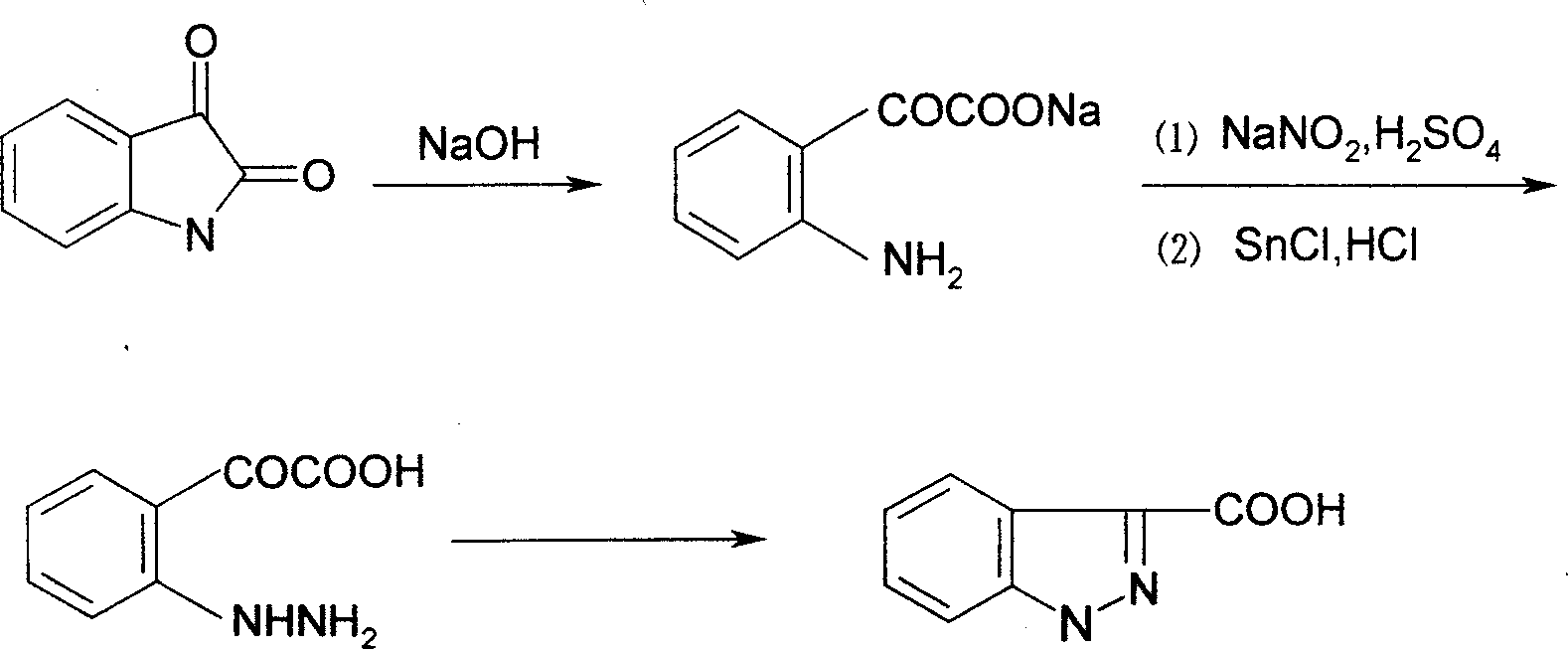
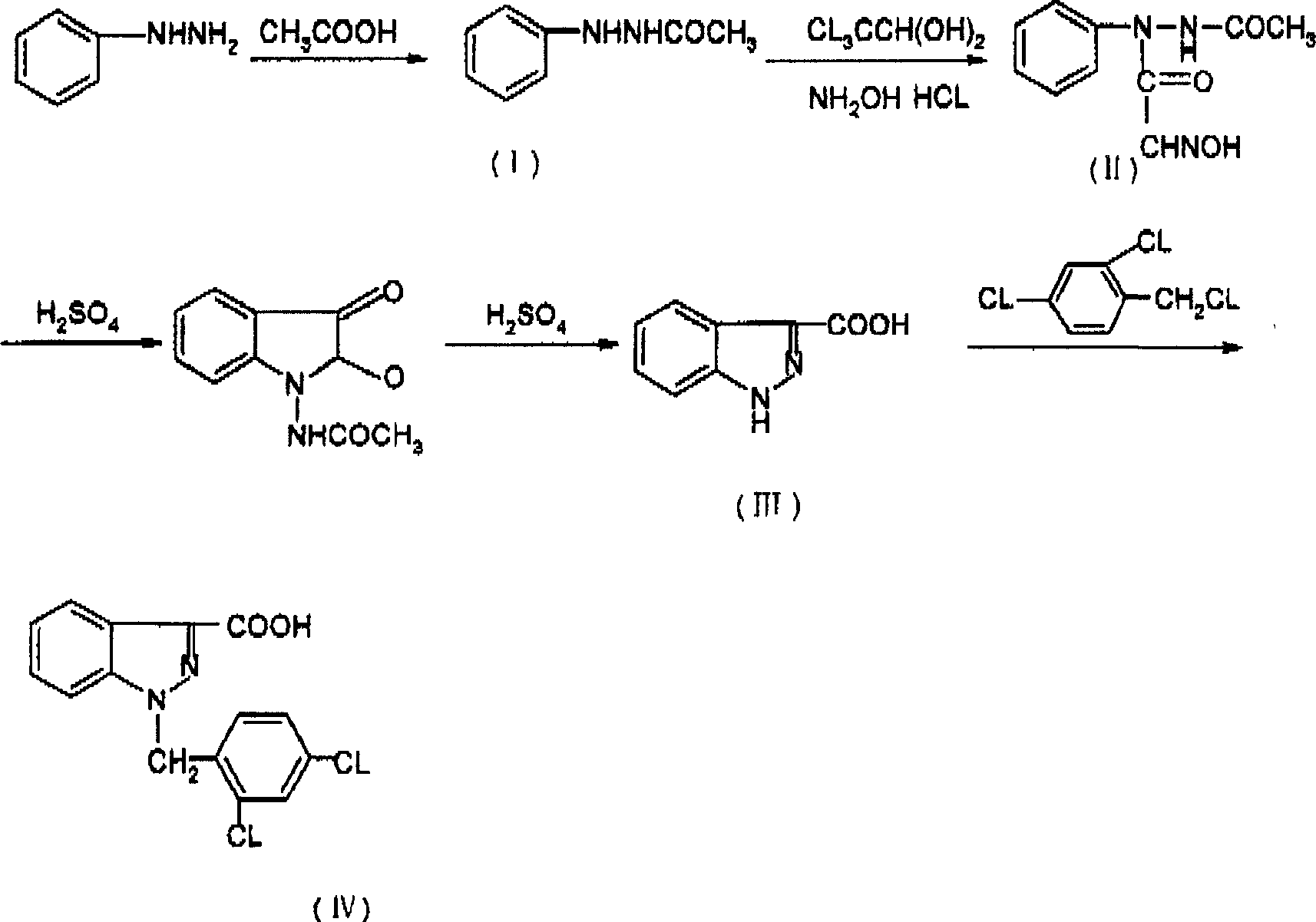
The invention relates to a process for synthesizing 1-[(2,4-dichlorobenzene) methyl]-1H-indazole-3-carboxyl acid which consists of, using phenylhydrazine as starting raw material, producing beta-acetylphenylhydrazine through reaction with glacial acetic acid, reacting with hydrated chloral and hydroxylamine hydrochloride, obtaining N-acetamido-isonitro-acetanilide, preparing 1H-indazole-3-carboxylic acid under the condition of concentrated sulfuric acid, finally subjecting 1H-indazole-3-carboxylic acid with 2,4-dichlorin benzyl chloride.
Owner:SHANGHAI ZHAOHUI PHARMA +1
Semi-bright nickel electroplating solution additive, semi-bright nickel electroplating solution and semi-bright nickel electroplating method
The invention provides a semi-bright nickel electroplating solution additive which comprises a brightener, a potential difference stabilizer, and a wetting agent; the potential difference stabilizer is chloral hydrate; the mass ratio of the brightener, the potential difference stabilizer, and the wetting agent is 5.3-123:1:0.7-35. The invention also provides a semi-bright nickel electroplating solution and a semi-bright nickel electroplating method. The semi-bright nickel electroplating solution additive of the invention provides stable potential difference environment for the electroplating solution, and is suitable for electroplating with high electroplating layer potential difference requirement and high workpiece corrosion resistance requirement.
Owner:HANGZHOU WIN WIN TECH CO LTD
Chloral hydrate permeabilization flaking method in microscopic identification of TCM
InactiveCN104764640AWide range of applicationsSuitable for teachingPreparing sample for investigationChloral hydrateFood science
The invention relates to a chloral hydrate permeabilization flaking method in microscopic identification of TC, which can effectively solve the problem of easy control, high efficiency and safety of chloral hydrate permeabilization. The method comprises the following steps: adding water in chloral hydrate for dissolving to obtain a chloral hydrate solution with mass concentration of 30-50%; adding 0.1-0.5g of traditional Chinese medicinal material powder into 2ml of chloral hydrate solution with mass concentration of 30-50% to prepare a mixture; filling the mixture into a container, preheating to 100-110 DEG C, then heating to 125-135 DEG C for 4-8 minutes, realizing permeabilization of the mixture; dropping the 1-2 drops of permeabilized mixture on a slide glass, adding 1-2 drops of dilute glycerin, uniformly mixing the traditional Chinese medicinal material powder on the slide glass, covering a coverslip, using an absorbent paper to remove overflowed liquid, and keeping the clean coverslip and slide glass. The flaking method can be effectively used for chloral hydrate permeabilization, and has the advantages of energy saving, safety, high efficiency, controllable performance, wide application and environmental protection, is especially suitable for teaching and scientific research, has strong utility value, and is great innovation of chloral hydrate permeabilization flaking method.
Owner:HENAN UNIV OF CHINESE MEDICINE
Licking brick for preventing and treating dairy cow ketosis
InactiveCN104757337APrevent volatilizationReduce usageHeavy metal active ingredientsHydroxy compound active ingredientsBrickDrug administration
The invention relates to a licking brick for preventing and treating dairy cow ketosis, relates to a dairy cow licking brick, and is used for solving the problems of being short in pharmaceutical effect lasting time, treated in symptoms, instead of root causes, high in recurrence rate and recurrence frequency and poor in palatability in the existing medicine for treating dairy cow ketosis. The licking brick for preventing and treating dairy cow ketosis is mainly composed of 20-30 g of chloral hydrate, 150-250 g of xylitol, 200-300 g of propylene glycol, 4-5 g of nicotinic acid, 25-35 g of nicotinic acid, 25-35 g of lysine, 40-60 g of methionine and 700-1000 g of molasses. According to the invention, dairy cow ketosis treatment components are prepared into the licking brick; and therefore, the licking brick has the advantages of being high in palatability, simple in drug administration manner, automatic for dairy cows to lick, manpower-saving and the like.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for detecting related substances in chloral hydrate
ActiveCN109406690AHigh sensitivitySensitive and accurateComponent separationSuppositoryCurative effect
The invention provides a method for detecting a chloral hydrate raw material and chloral hydrate suppository related substances. The method comprises the steps of carrying out derivatization reactionand then carrying out GC-MS detection. The method is good in specificity and high in sensitivity, the stability and repeatability of the method are good, the analyzing efficiency is high, various impurities of chloral hydrate suppository can be qualified and quantified sensitively and accurately, therefore, the quality of the chloral hydrate suppository is evaluated objectively, comprehensively and accurately, and the method plays an important significance on control over the quality of the chloral hydrate suppository and guaranteeing the clinical effects.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD +1
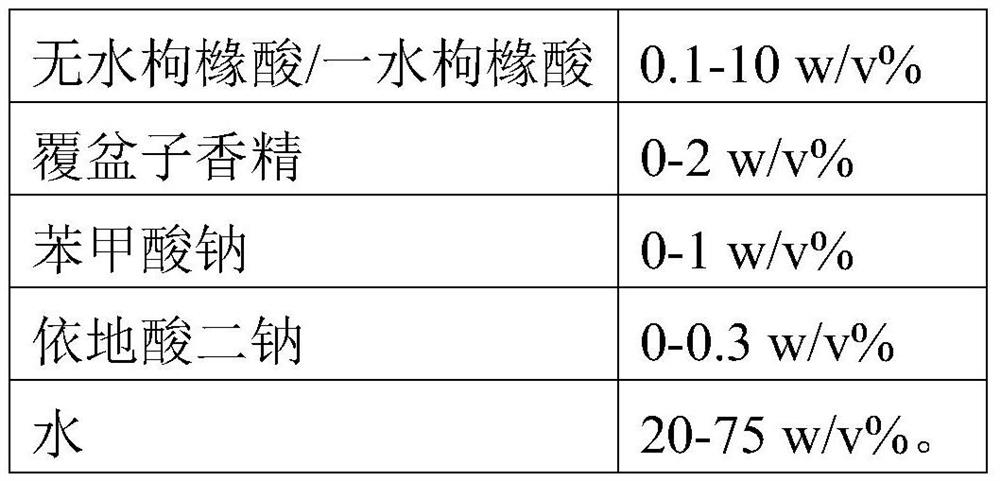
Oral sedative for children
ActiveCN104800154AEasy transferRaise the extramembrane voltageNervous disorderHydroxy compound active ingredientsSucroseSIMPLE SYRUP
The invention discloses an oral sedative for children. The oral sedative comprises the following components: simple syrup, a chloral hydrate solution with the mass concentration of 10%-20%, and a glucose solution, containing sodium citrate, with the mass concentration of 5%-10% according to the volume proportion of the (1-2):2:1, wherein the mass concentration of the glucose solution dissolving sodium citrate is 5%-10%; the main simple syrup component is the simple syrup of cane sugar, and the mixed oral sedative is a weak alkaline solution with the pH value of 7-8. According to the sedative formula of the application, (1) the taste of the conventional chloral hydrate is improved, and the conflict reaction when the sedative is taken by an infant patient is reduced; (2) the effect-taking time of the oral chloral hydrate sedative is shortened, so that the sedative can be absorbed by small intestines relatively easily, the bioavailability is improved, the stimulation on gastrointestinal tracts is reduced, and nausea and vomiting are reduced.
Owner:赵柏松
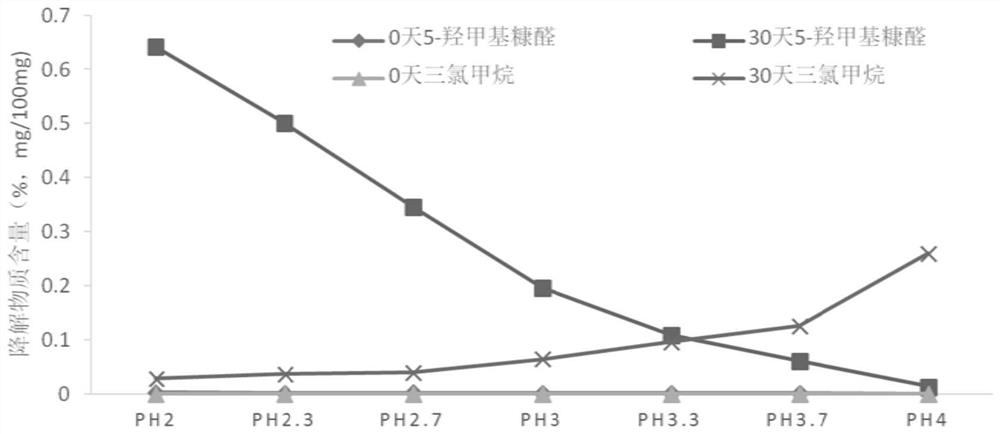
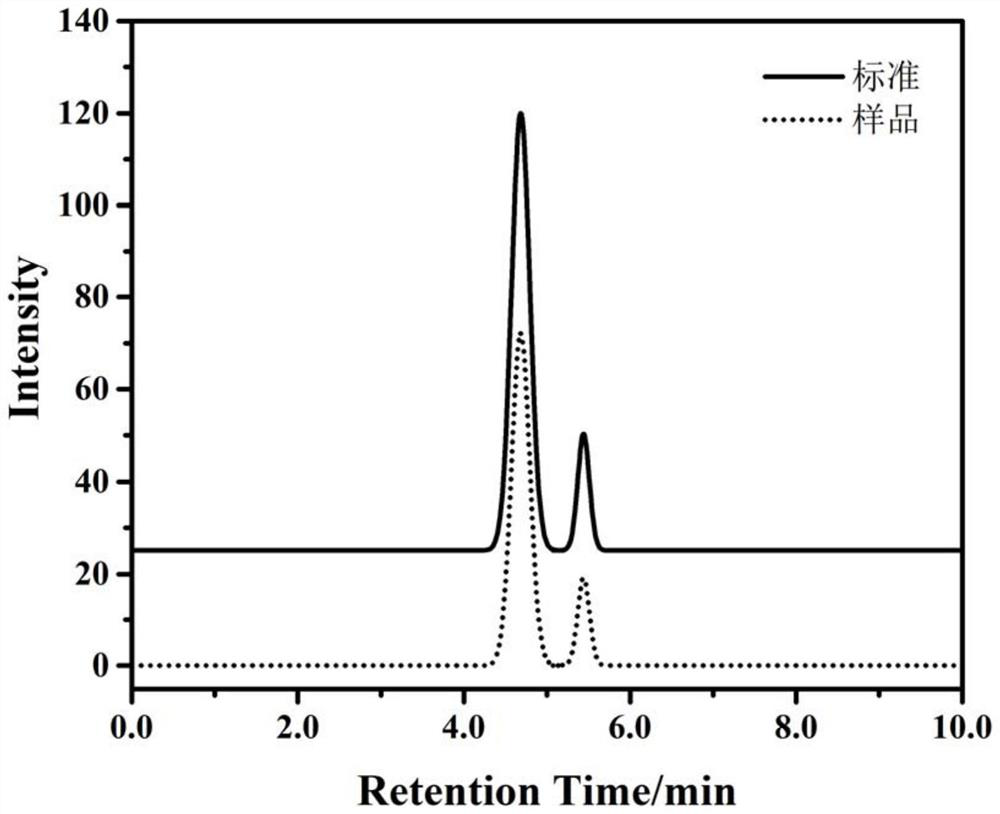
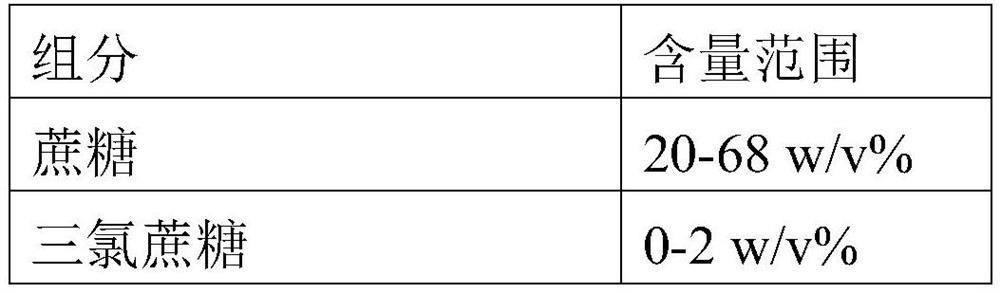
Stable chloral hydrate solution and preparation method and application thereof
ActiveCN110151687AIncrease concentrationImprove stabilityNervous disorderDispersion deliveryChemistryCitric acid
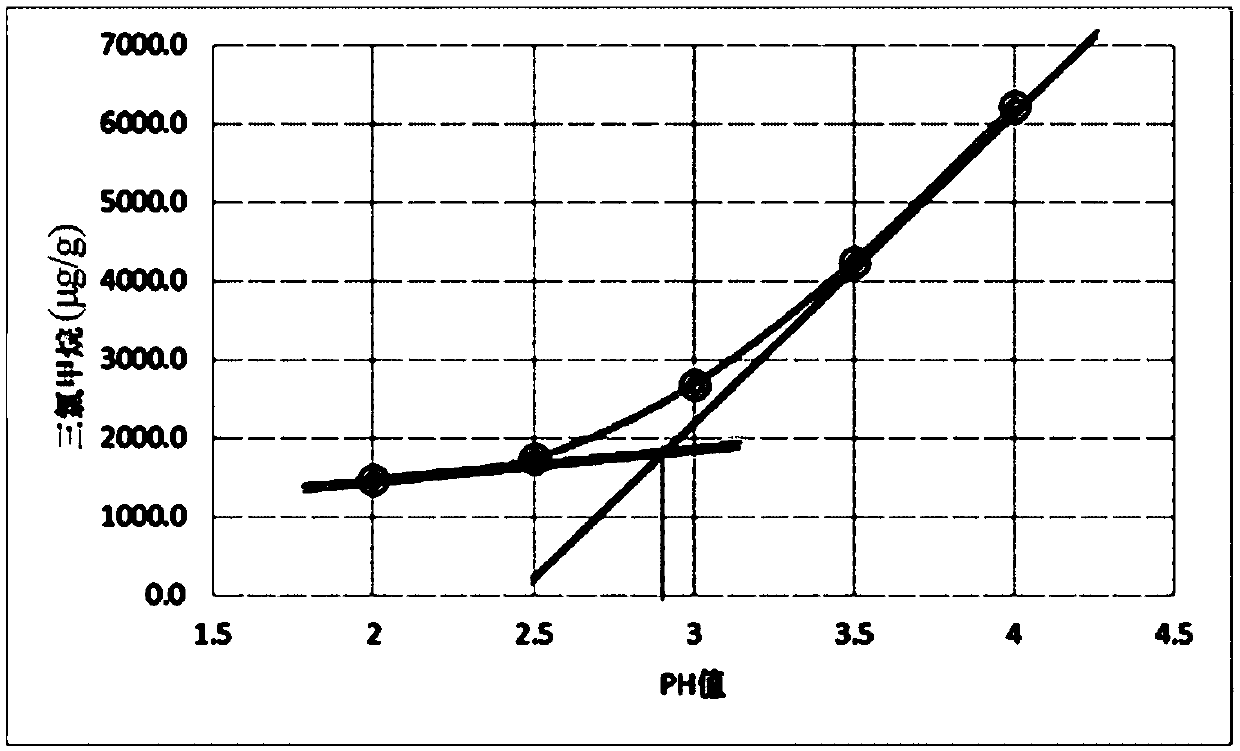
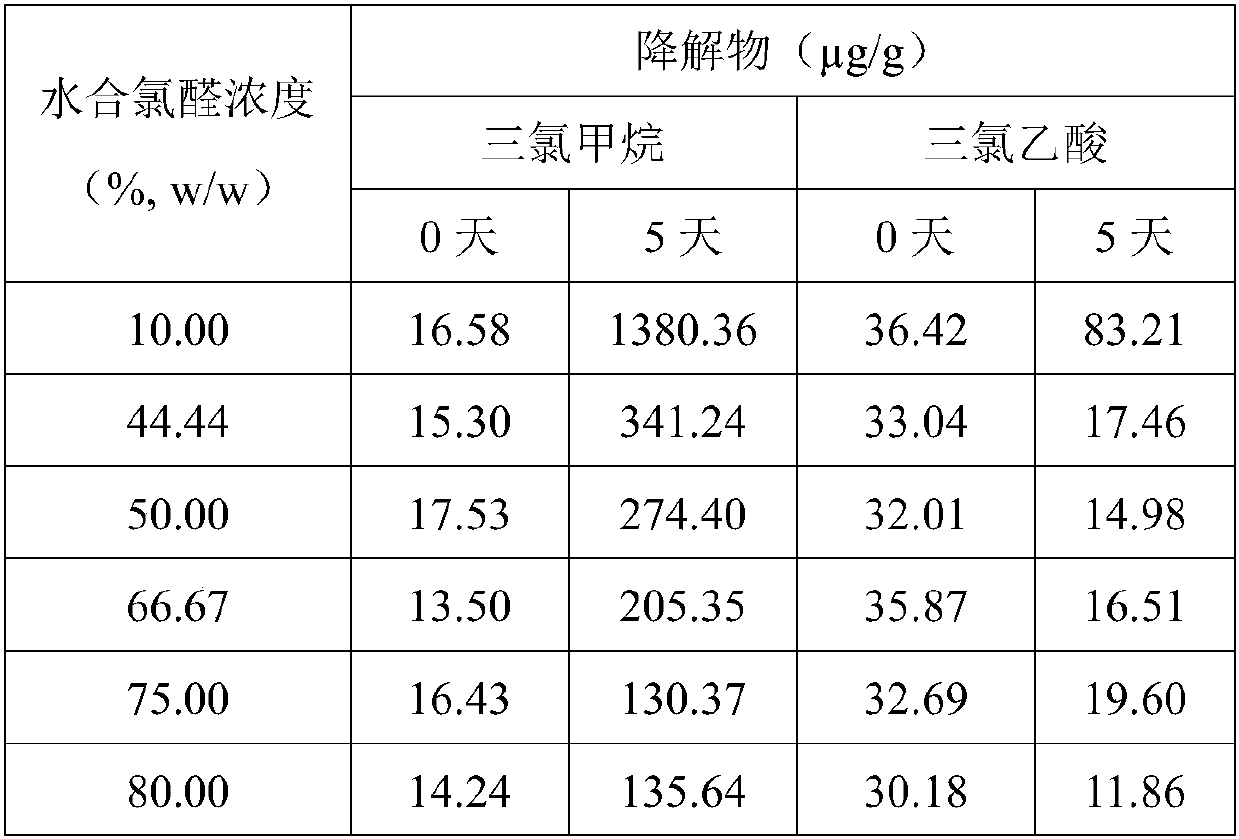
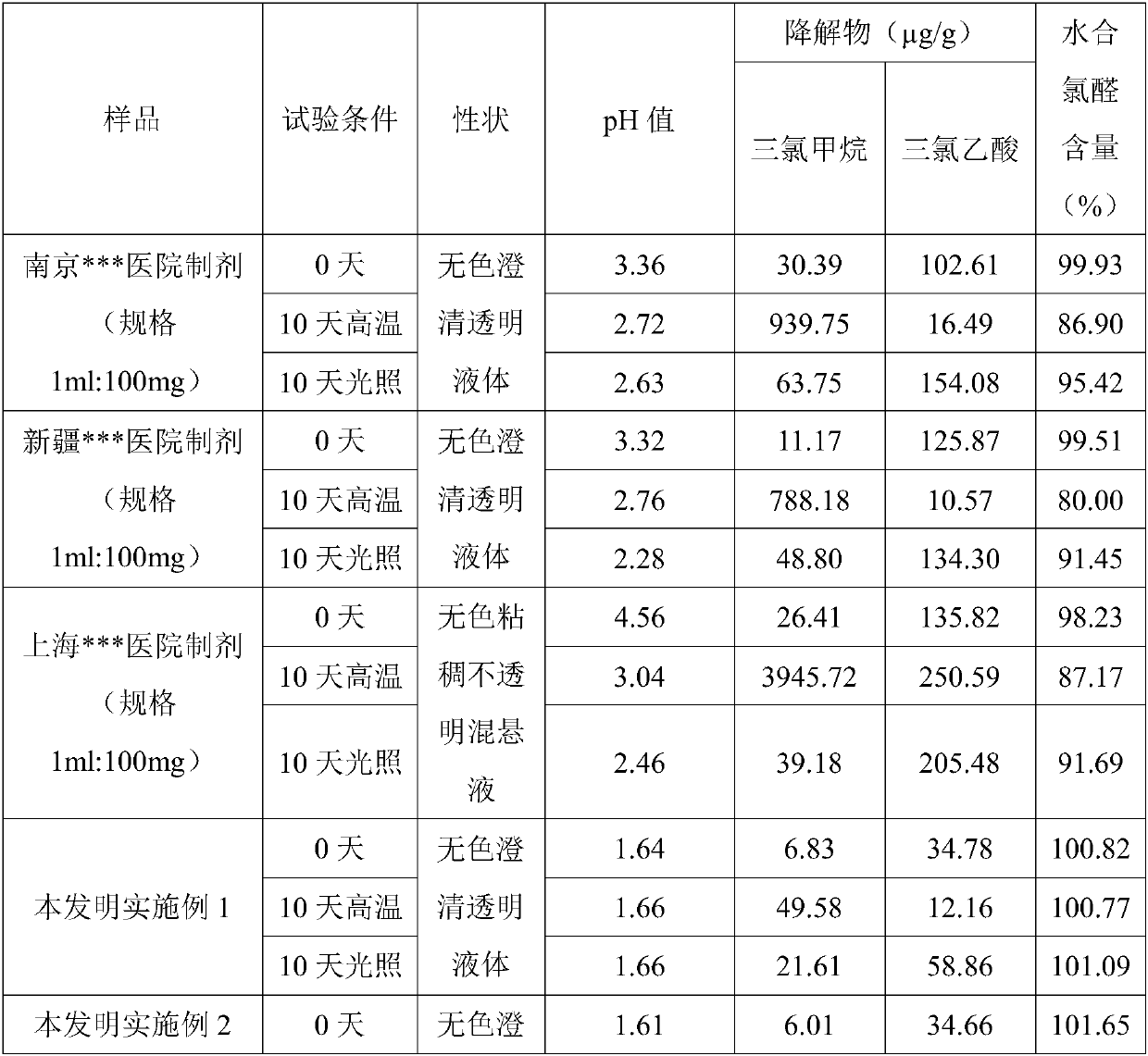
The invention discloses a chloral hydrate solution. The chloral hydrate solution comprises chloral hydrate, pharmaceutically-acceptable acid and water, wherein the mass concentration of the chloral hydrate is 40-80%, and the pH value of the chloral hydrate solution is preferably 1.0-2.9. Particularly, the invention relates to a stable chloral hydrate solution. The stable chloral hydrate solution comprises chloral hydrate, monohydrate or anhydrous citric acid and purified water, wherein the mass concentration of the chloral hydrate is 44-80%, and the pH value of the chloral hydrate solution ispreferably 1.0-2.9. The invention further relates to a preparation method and application of the chloral hydrate solution.
Owner:TEFENG PHARM CO LTD
Method for preparing single-cell suspension of aortic cells of mouse
InactiveCN112210533AMany immune cellsEfficient manufacturing methodCell dissociation methodsBlood/immune system cellsIntraperitoneal routeBlood Vessel Tissue
The invention discloses a method for preparing a single-cell suspension of aortic cells of a mouse. The method is characterized by specifically comprising the following steps of firstly, preparing 1 ml of an enzyme mixed solution, the surviving healthy wild mouse, an operating table of which the whole tabletop is a flat ice surface and a plurality of instruments; and then, performing intraperitoneal injection anesthesia on the prepared wild mouse by 200-500 microliters of chloral hydrate until the mouse is dead, immediately performing perfusion flushing on blood vessels of the wild mouse by 20ml of a phosphate buffer salt solution, performing overall disinfection on the dead wild mouse by alcohol with the volume fraction of 75%, performing thoracotomy andlaparotomy after disinfection is completed, and exposing the heart and the aorta. The whole operation process is completed on the operating table. Preparation of a single-cell suspension of vascular tissues of the mouse by using a shearing and mixed enzyme combined digestion process is simple, convenient and feasible, more immune cells exist, and an effective tissue single-cell suspension preparation method is provided for research of vascular immune cells.
Owner:ANHUI NORMAL UNIV
Stable chloral hydrate syrup as well as preparation method, quality control method and application thereof
PendingCN112656758ADesign scienceClever thinkingNervous disorderComponent separationSucroseChloral hydrate
The invention discloses stable chloral hydrate syrup as well as a preparation method, a quality control method and application thereof, belongs to the technical field of medicines, and solves the problems that chloral hydrate is poor in stability, harsh in storage condition and short in validity period and needs to be diluted during use in the prior art. The stable chloral hydrate syrup comprises chloral hydrate, cane sugar and water, the mass volume concentration of chloral hydrate is 10%, and the pH value of the chloral hydrate syrup is 2.3-3.0. The chloral hydrate syrup disclosed by the invention also comprises glycerol. The chloral hydrate syrup disclosed by the invention adopts the specification that the concentration of chloral hydrate used clinically is 10%, does not need to be newly prepared when in use, and is accurate in dosage, safe, controllable and stable in property.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD +1
Refrigerator deodorants
InactiveCN104258439AEffectively removes odorsEliminate odorBiocideDisinfectantsGlycerolRefrigerated temperature
The invention relates to refrigerator deodorants. The refrigerator deodorants comprise, by weight, 3-8 parts of sodium carbonate, 2-4 parts of chloral hydrate, 4-7 parts of potassium permanganate, 3-5 parts of ethyl alcohol, 1-3 parts of single ricinoleic acid glyceride, 2-6 parts of sodium chloride, 1-5 parts of ten-polyglycerol palmitate, 2-4 parts of glycerinum, 0.6-2 parts of lactic acid and 25 parts of water. The refrigerator deodorants have the beneficial effects of being colourless and tasteless, free of corrosion, environment-friendly and the like; meanwhile, peculiar smells in a refrigerator can be effectively removed; in addition, a certain bactericidal effect is achieved.
Owner:QINGDAO TORIX ELECTRONICS TECH
Methods for detection of chloral hydrate in dichloroacetic acid
InactiveUS7173123B2Accurate measurementSugar derivativesMicrobiological testing/measurementAcetic acidChloral hydrate
Owner:IONIS PHARMA INC
Method for determining content of halogenated acid in chloral hydrate or preparation thereof
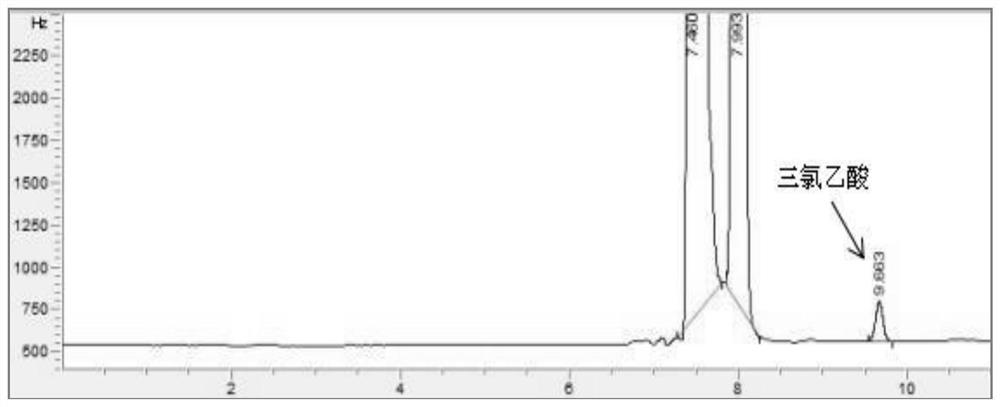
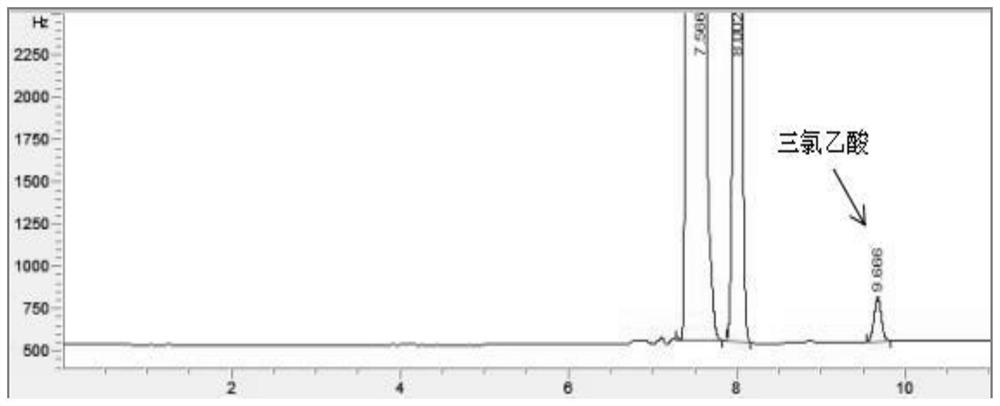
ActiveCN112345651AAvoid factors that interfere with test resultsEasy to operateComponent separationPhysical chemistryVapor phase chromatography
The invention relates to a method for detecting the content of impurities in a drug, in particular to a method for determining the content of halogenated acid in chloral hydrate or a preparation thereof through gas chromatography.
Owner:TEFENG PHARM CO LTD
Preparation method of acetic acid inducted rat individual same-size gastric ulcer model
The invention relates to a manufacturing method of a single and isometric gastric ulcer model induced by acetic acid in a rat. The method comprises procedures as follows: the rat is fasting but drinks freely for 24 hours; the chloral hydrate of 10 percent (0.3nl / 100g weight) is used for anesthetizing the rat by muscle administration; lodophor is used for sterilizing the abdomen skin of the rat; a cut is made on the side wall of the abdomen and a stomach is pulled out slightly; the nipper with an annular front end (with the inner diameter of 9mm) clamps a corpus ventriculi part 3mm away from a pyloric part; after a distal stomach-antrum is penetrated, 0.81ml of the mineral oil and the acetic acid solution of 160 percent of 0.02ml ( with the total volume of 0.2ml) are injected into a lumen; after 45 seconds, the mixed solution of acetic acid and the mineral oil is absorbed from stomach; afterwards, the physiological saline of 2ml is injected into a gastral cavity for flushing and is drained off after 1min; and at last, the stomach is put back and the abdomen is sewed up. After an operation, the rat is carried out usual care. The method is easy to be handled and can make a single round ulcer with the inner diameter of 9mm. The area and the depth of the ulcer can be controlled. In addition, the problem of adhesion can be solved; the ulcer is extremely similar with the human ulcer in pathological features and healing mechanism; and the ulcer model is very sensitive to various anti-ulcer drugs.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Administration method and administration device for experimental animal brain
InactiveCN104274255ASolve the problem of relatively large physical damageEasy injectionVeterinary instrumentsHeterocyclic compound active ingredientsDrugs solutionPenicillin
The invention discloses an administration method for an experimental animal brain; by adopting a method for burying a catheter into the experimental animal brain, injection to the experimental animal brain is more convenient. According to the administration method, a special successive administration device is adopted, and a mouse or a rat is taken as an experimental animal, and the administration method comprises the following steps: 1, narcotizing the experimental animal with 30 percent of chloral hydrate, fixing the experimental animal on a mouse brain locator, and determining the position of a paracele; 2, burying an administration catheter into the paracele of the experimental animal according to the step, and fixing the catheter to the head of the experimental animal by dental cement when cerebrospinal fluid overflows from the catheter; 3, in 4 days after the catheter is buried successfully, performing successive intramuscular injection every day with 0.2ml of 0.8 million units of penicillin sodium, and diminishing inflammation of the experimental animal; 4, performing administration: sucking up a right amount of to-be-experimented drug solution by a 10mul microinjector, and partially inserting the syringe needle of a microsyringe into the catheter buried into the experimental animal brain, wherein the insertion depth is 0.5-2.0cm, and successive administration can be performed on the mice brain from the catheter.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of bacillus cereus polyclonal antibodies
InactiveCN103804494AHigh potencyStrong specificitySerum immunoglobulinsImmunoglobulins against bacteriaWater bathsAdjuvant
The invention provides a preparation method of bacillus cereus polyclonal antibodies, which comprises the following steps: 1, enrichment: coating activated bacillus cereus on a flat plate for enlarge cultivation; 2, fixation: fixing surface compositions of the bacillus cereus by using a modified boiling water bath method, so that antigenic determinants are fully exposed; 3, emulsification: preparing a uniform emulsified liquid from an adjuvant-bacillus mixture by using a self-assembled emulsifier; 4, immunization: carrying out animal immunization in a mode of being based on multiple loca on the back and supplemented by peripheral loca; 5, antiserum titer: evaluating the antiserum titer by using an ELISA method, and carrying out enhanced immunization; 6, taking whole blood: taking whole blood from cervical arteries under the condition of anesthesia by using chloral hydrate; 7, preparation of antiserum: standing the rabbit whole blood at optimum temperature so as to carry out natural chromatography, and carrying out centrifugal separation on the antiserum; and 8, purification: purifying the antiserum by using an n-caprylic acid-ammonium sulfate method, so that a polyclonal antibody is prepared. The polyclonal antibody prepared by using the preparation method disclosed by the invention is high in titer, good in specificity and strong in sensitivity, and has a great application advantage.
Owner:FUDEAN OF BEIJING TECH +1
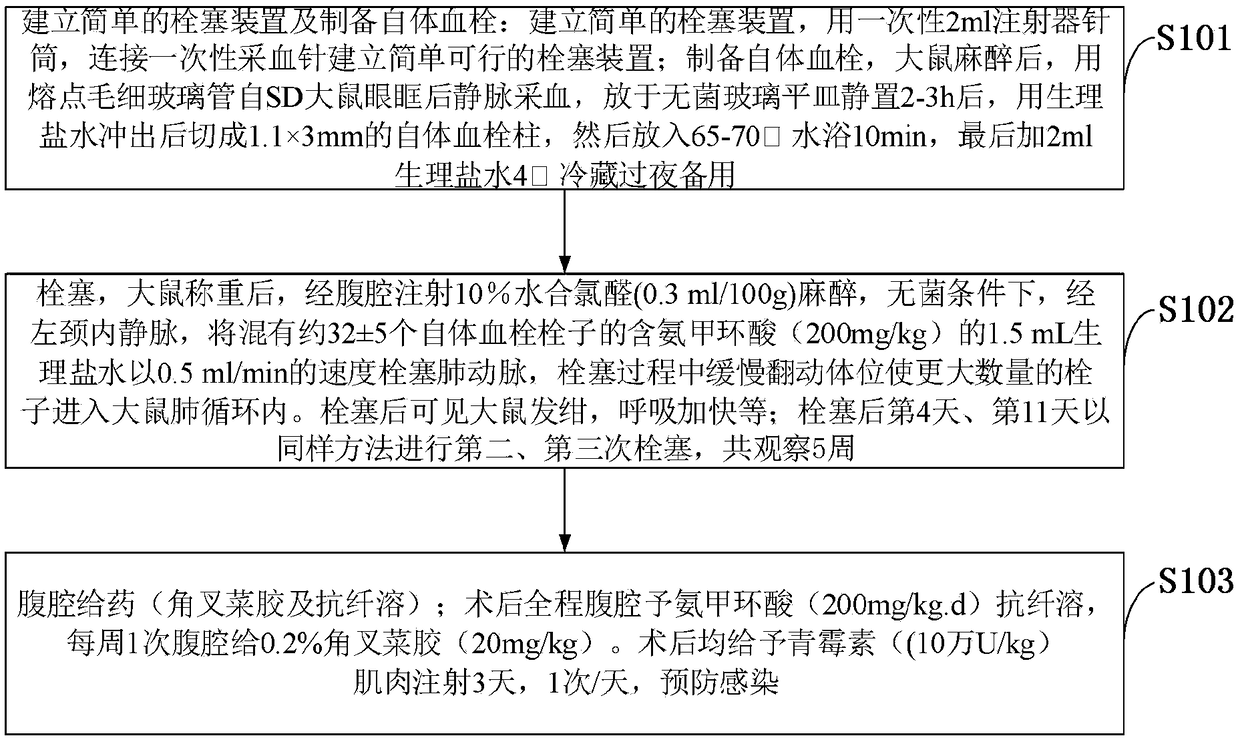
Chronic thromboembolic pulmonary hypertension rat model and building method thereof
The invention belongs to the technical field of a new variety of vertebrates, and discloses a chronic thromboembolic pulmonary hypertension rat model and a building method thereof. Chloral hydrate ata concentration of 10 percent is injected through the abdominal cavity for anesthesia; under a sterile condition, through a left internal jugular vein, 1.5 mL of tranexamic acid-containing normal saline mixed with about 32 + / - 5 autologous thromboemboli embolizes the pulmonary artery at a rate of 0.5 ml / min; the rat is slowly turned in the embolizing process to enable a larger number of emboli toenter the lung circulation of the rat; after being embolized, the rat has cyanosis and breathes faster; on the fourth day and the eleventh day of the embolization, the rat is embolized for the secondtime and the third time according to the same method; the rat is observed for 5 weeks. The CTEPH model built by the building method disclosed by the invention takes the autologous emboli of the rat asemboli to embolize the pulmonary artery and enables the autologous emboli to embolize a blood vessel as long as possible under the help of pro-inflammatory effect of carrageenin, so as to really model the whole pathophysiological process of pulmonary embolism secondary CTEPH of a clinical CTEPH patient.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Methods for detection of chloral hydrate in dichloroacetic acid
InactiveUS20050113569A1Accurate measurementSugar derivativesMicrobiological testing/measurementAcetic acidChloral hydrate
Owner:IONIS PHARMA INC
Method for constructing temporomandibular joint osteoarthritis animal model by applying digital technology
InactiveCN113440254AAvoid heterogeneityAvoid disadvantagesComputer-aided planning/modellingModel sampleZinc Phosphate Cement
The invention discloses a method for constructing a temporomandibular joint osteoarthritis animal model by applying a digital technology, relates to the technical field of medicine and animal models, and solves the problem that an existing animal model construction method is poor in intuition, poor in precision and poor in expected controllability, resulting in that a model sample is easy to generate heterogeneity. The method for constructing the temporomandibular joint osteoarthritis animal model by applying the digital technology comprises the steps of: selecting an SD rat, anesthetizing, and digitally scanning animal teeth; selecting a 6-week-old female SD rat with the weight of 160-180 g, feeding the rat in a clean environment, adaptively feeding the rat for one week, injecting 10% chloral hydrate into the abdominal cavity of the SD rat, and anesthetizing the rat; producing a telescopic crown; establishing the temporomandibular joint osteoarthritis animal model; and under the anesthesia of chloral hydrate, adhering by zinc phosphate cement. The method applies the digital technology, is simple in production process, more visual in 3D scanning and high in precision, has predictability, and avoids heterogeneity of samples.
Owner:JIAMUSI UNIVERSITY
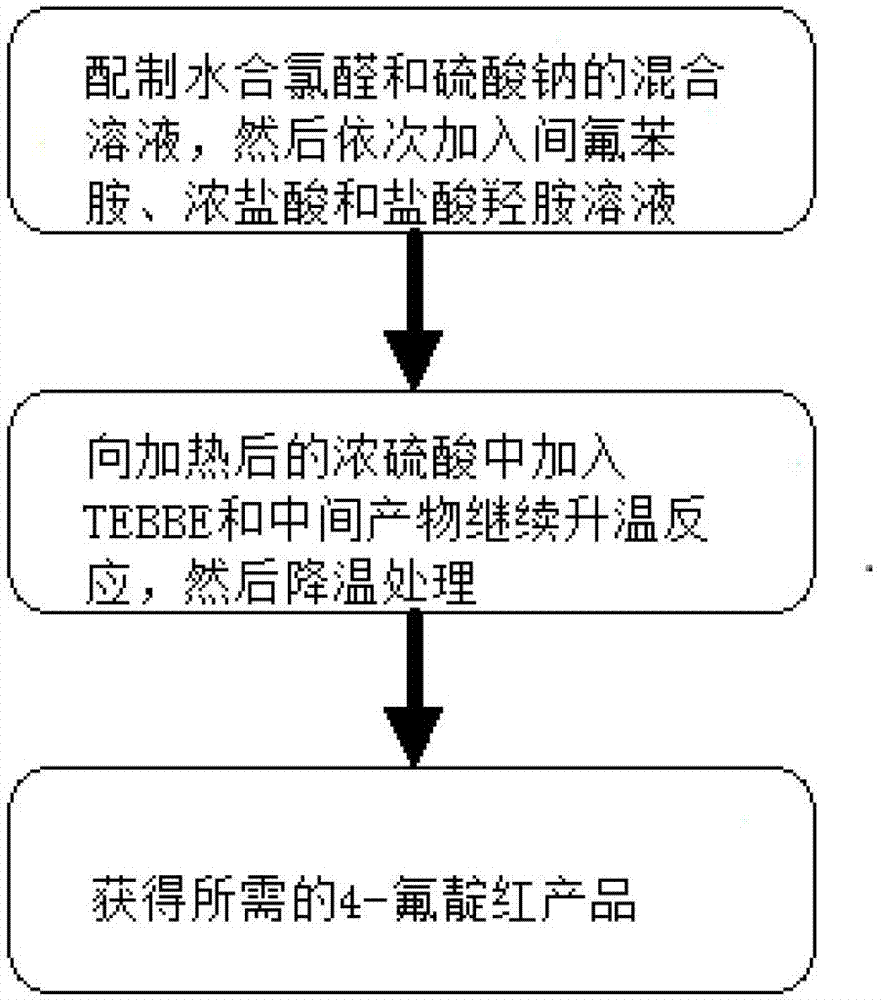
Industrialization preparation method for 4-fluoroisatin and product thereby
ActiveCN103319395ASimple processImprove quality controlOrganic chemistryHydroxylamine HydrochlorideControl quality
An industrialization preparation method for 4-fluoroisatin is disclosed by the invention, and comprises: (a) dissolving chloral hydrate and sodium sulfate in water, heating to 30 DEG C-35 DEG C, then successively adding m-fluoroaniline, concentrated hydrochloric acid and an aqueous solution of hydroxylamine hydrochloride, heating to 80 DEG C-90 DEG C for continually reaction, after a complete reaction, air-pump filtering and washing the filter cake with water, and obtaining the intermediate; and (b) heating concentrated sulfuric acid to 50 DEG C-60 DEG C, successively adding TEBBE reagent and the intermediate obtained in step (a), then continually heating to 80 DEG C-85 DEG C and reacting, after a complete reaction, cooling and precipitating an orange red precipitate, and therefore obtaining the 4-fluoroisatin product. The invention also discloses the corresponding 4-fluoroisatin product. The industrialization preparation method can help to obtain the product with a simple process, a high yield and low cost, and is convenient to control quality and applicable in industrialization production in a large scale.
Owner:WUHAN LUOHUA TECH
Production method of isatin
The invention relates to a production method of isatin. The production method comprises the following steps: (1) aniline hydrochloride (or containing part of un-salinized aniline) produced by reactionof aniline and hydrochloric acid is subjected to oximation reaction with chloral hydrate, hydroxylamine hydrochloride, water and sodium sulfate decahydrate or sodium sulfate in an acid medium, oximido acetanilide is obtained in the range of the ratio determined by the action mechanism of sodium sulfate, part of solid-phase sodium sulfate is retained in the system in the initial stage of the reaction, and the ratio of aniline (mol) / water (kg) can be increased; (2) in the closed-loop reaction, the reaction time is prolonged in exchange for low reaction temperature and low sulfuric acid concentration in order to improve the reaction safety and increase the yield of isatin. Compared with typical processes, the method has the advantages that the reaction safety is improved, the yield of isatinis increased, unit consumption and production cost can also be reduced, the utilization rate of equipment is increased, and the amount of produced waste water is reduced. According to the process, noobvious amplification effect is found by tests in thousands of moles.
Owner:CHANGZHOU UNIV
New use of chloral hydrate for preparing pulmonary fibrosis disease
The invention discloses a new application that chloral hydrate is used for preparing drugs of pulmonary fibrosis diseases, which pertains to the medical field. The invention aims at providing the new application of the chloral hydrate, which particularly relates to the new application that the chloral hydrate is used for preparing the drugs of the pulmonary fibrosis diseases. The application of the chloral hydrate overcomes the difficulty of pulmonary fibrosis and causes the diseases that are similar to cancers to be controlled, thereby saving the lives of patients with the pulmonary fibrosis diseases.
Owner:吉林大学第二医院
A method for detecting related substances in chloral hydrate
ActiveCN109406690BHigh sensitivitySensitive and accurateComponent separationClinical efficacyPhysical chemistry
The invention provides a method for detecting related substances of chloral hydrate raw material and chloral hydrate plug, including derivatization reaction, and then performing GC-MS detection, the method has good specificity, high sensitivity, good method stability and repeatability, and analysis efficiency High, sensitive and accurate qualitative and quantitative impurities in chloral hydrate suppositories, so as to objectively, comprehensively and accurately evaluate the quality of chloral hydrate suppositories, which is of great significance for controlling the quality of chloral hydrate suppositories and ensuring clinical efficacy.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD +1
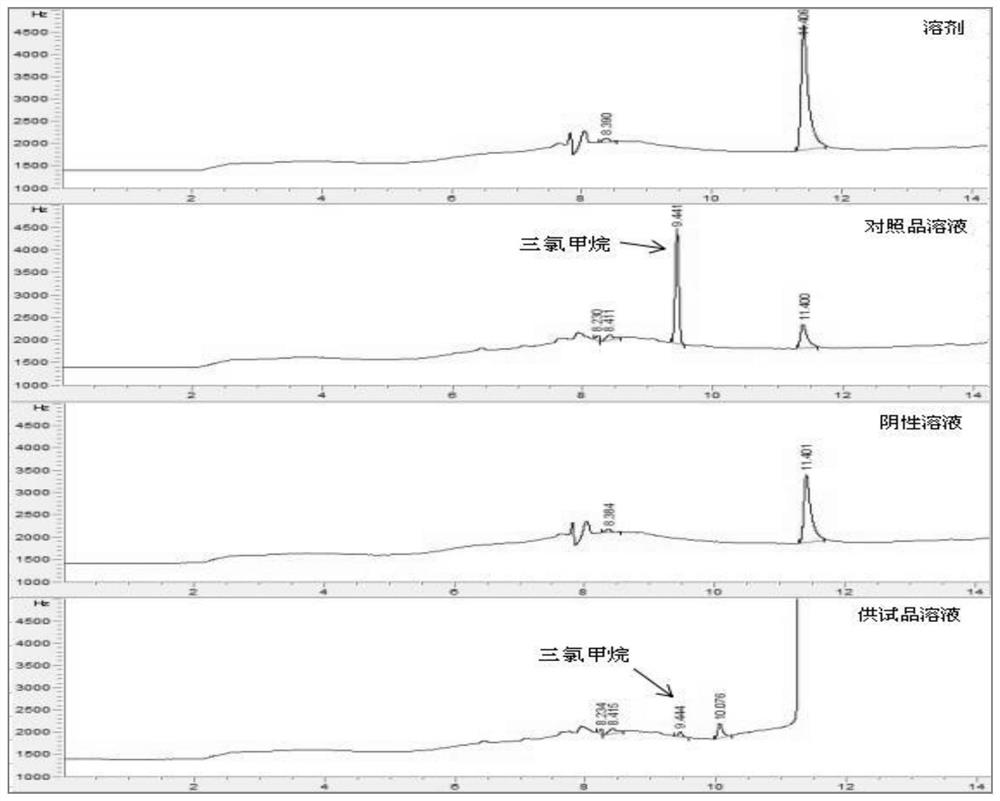
Method for determining the content of chlorinated alkanes in chloral hydrate or its preparations
ActiveCN112014487BAvoid degradation and interference with assay resultsEasy to operateComponent separationAlkaneInorganic salts
The present invention provides the method for measuring the content of chloralkane in chloral hydrate or its preparation by gas chromatography, it comprises the following steps: the first step: accurately weigh chlorinated alkane reference substance, it is dissolved in organic solvent, obtains Reference substance solution; the second step: the sample chloral hydrate or its preparation to be tested is optionally dissolved or diluted with water or an inorganic salt solution, and an organic solvent is added for extraction, and the organic layer is taken as a test solution; and the third step: by gas phase Chromatography was used to detect the reference solution and the test solution, and calculate the content of chlorinated alkanes.
Owner:TEFENG PHARM CO LTD
Sample pretreatment method for detecting content of chloral hydrate in health care product and detection method of chloral hydrate
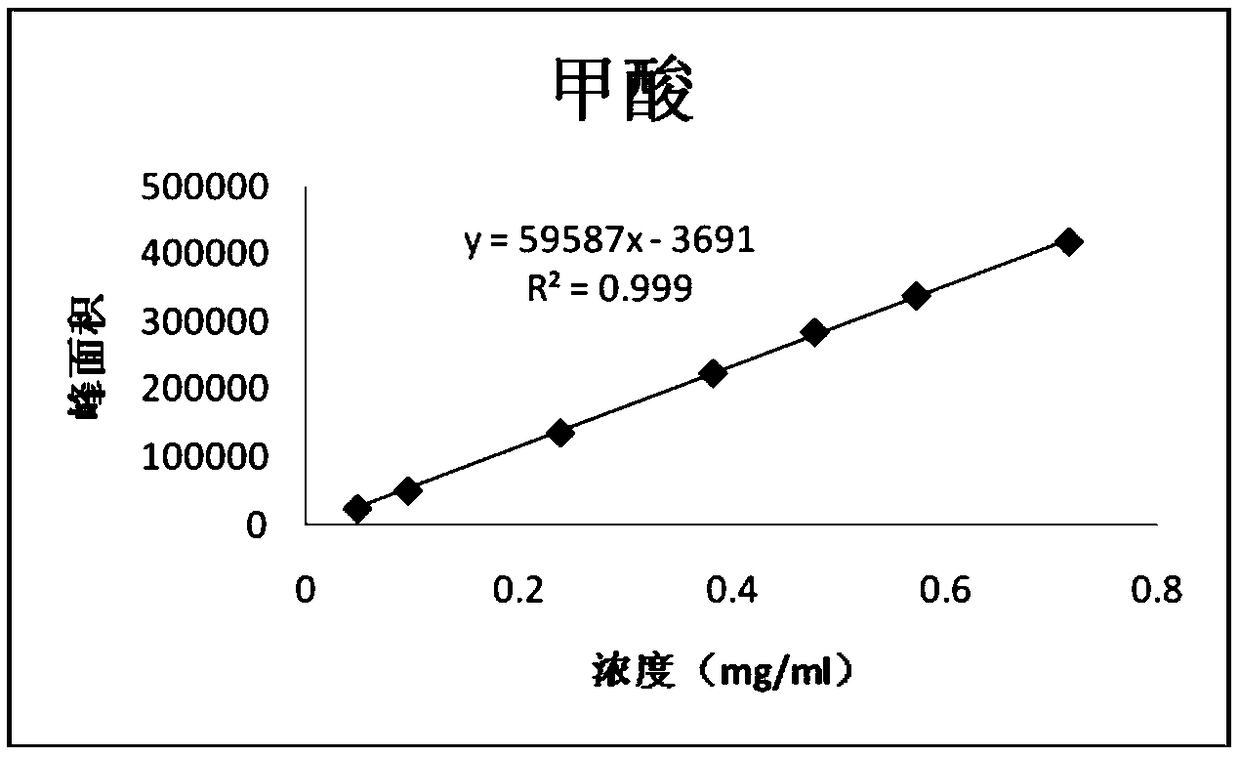
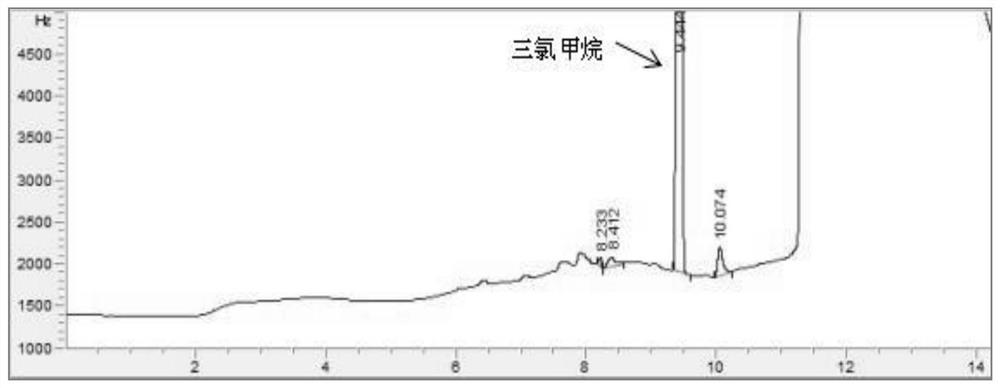
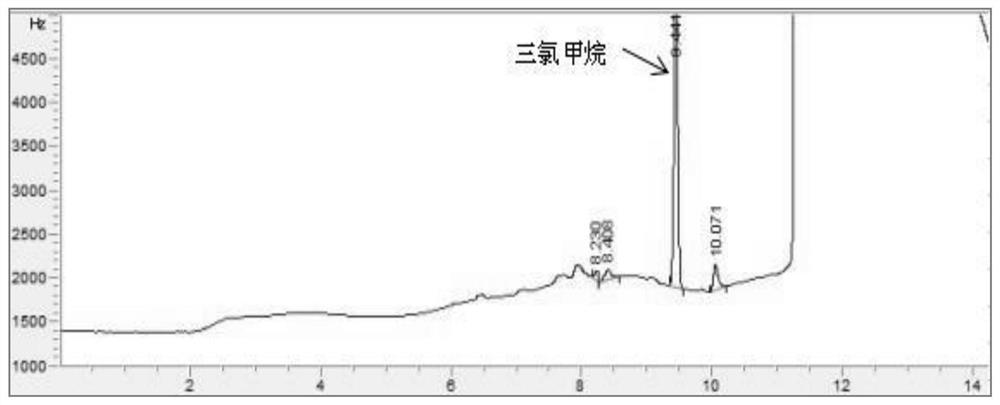
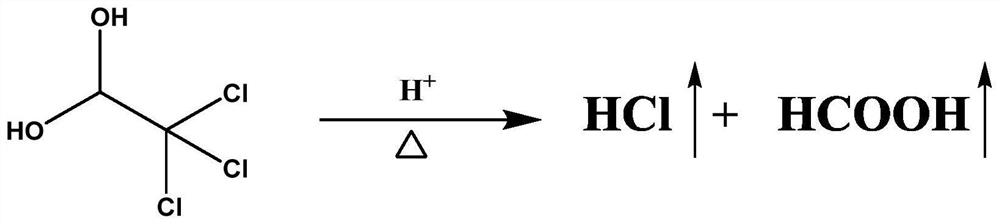
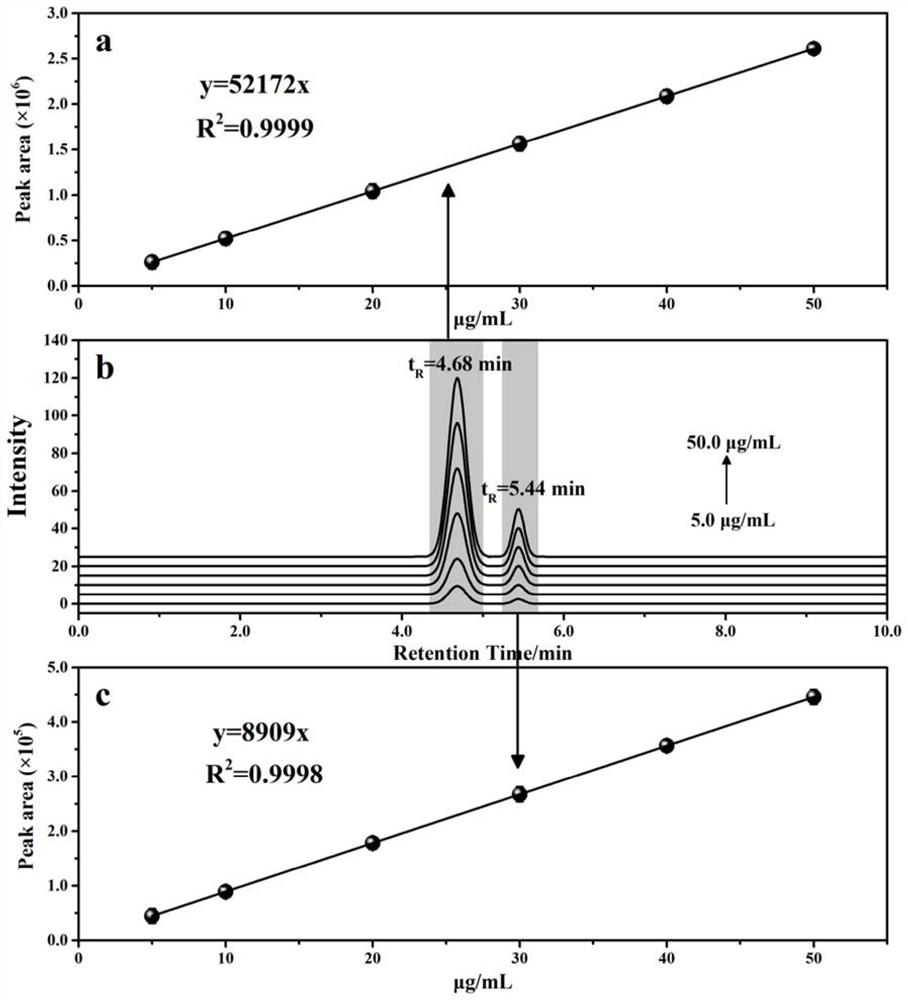
PendingCN114705768AReduce processing timeSimplify the detection and analysis processComponent separationSeparation technologyChloride
The invention relates to a sample pretreatment method for detecting the content of chloral hydrate in a health-care product and a detection method of the chloral hydrate. The sample pretreatment method for detecting the content of chloral hydrate in the health care product comprises the following steps: taking a proper amount of sample, adding an acid solution, heating, and decomposing chloral hydrate to generate HCl and HCOOH gases; and absorbing the generated gas with water to obtain a solution to be detected. According to the method, chloral hydrate can be subjected to a hydrolysis reaction under acidic and heating conditions and generate hydrogen chloride and formic acid with high volatility, then the hydrogen chloride and the formic acid are separated by combining a gas-liquid separation technology, and the content of chloral hydrate in the health care product is determined by adopting an analysis technology for detecting chloride ions or formic acid. Compared with the prior art, the sample pretreatment method for detecting the content of chloral hydrate in the health care product and the detection method of the sample pretreatment method have the advantages that matrix interference can be effectively eliminated, the sample pretreatment time is shortened, the detection and analysis process of chloral hydrate in the health care product is greatly simplified, the steps are simple, and the sensitivity is high.
Owner:GUILIN MEDICAL UNIVERSITY
Method for determining content of chloroalkane in chloral hydrate or preparation thereof
ActiveCN112014487AAvoid degradation and interference with assay resultsEasy to operateComponent separationAlkaneInorganic salts
The invention provides a method for determining the content of chloroalkane in chloral hydrate or a preparation thereof by gas chromatography, which comprises the following steps of 1, precisely weighing a chloroalkane reference substance, and dissolving the chloroalkane reference substance in an organic solvent to obtain a reference substance solution, 2, dissolving or diluting a to-be-detected sample chloral hydrate or a preparation thereof with water or an inorganic salt aqueous solution optionally, adding an organic solvent for extraction, and taking an organic layer as a test solution, and 3, respectively detecting the reference substance solution and the test solution through gas chromatography, and calculating the content of the chloralkane.
Owner:TEFENG PHARM CO LTD
Calming spray used before live pig slaughtering and preparation method thereof
InactiveCN105616872AReasonable ratioAvoid physical impactHeavy metal active ingredientsNervous disorderBiotechnologySucrose
The invention provides a calming spray used before live pig slaughtering, relating to the technical field of live pig slaughtering. The calming spray comprises the following raw materials: 80-100 parts of chloral hydrate solution, 60-70 parts of sodium citrate, 60-80 parts of glucose solution, 5-7 parts of sucrose, 6-8 parts of simple syrup, 3-5 parts of vermilion powder, 6-8 parts of magnetite powder, 7-9 parts of euphorbia royleana, 6-8 parts of seed of wild jujube, 3-5 parts of amber essential oil, 2-4 parts of polygala root, 3-5 parts of tuckahoe, 4-6 parts of cortex albiziae and 60-80 parts of water. The calming spray has the beneficial effects that the adopted raw materials are rich and cheap, the preparation process is simple and convenient, the raw material ratio is reasonable, the raw materials are easy to obtain, the used raw materials are pollution-free, and non-toxic chemical agents and Chinese patent medicines are reasonably compounded, thereby ensuring the lasting and safe drug effect, avoiding the strong body influence to live pigs, being harmless to the meat quality of the live pigs, and ensuring the emotional stability of the live pigs.
Owner:WUHE YONGMING FOOD CO LTD
Garden seed sterilization mildew-proof treating agent
InactiveCN105123799AHelps growImprove survival ratePlant growth regulatorsBiocideSodium acetatePolythylene glycol
The invention relates to a garden seed sterilization mildew-proof treating agent. The treating agent is composed of the following ingredients, by weight, 28-32 parts of corn starch, 30-34 parts of red peppers, 26-30 parts of anhydrite mine, 30-34 parts of fly ash, 26-30 parts of clinoptilolite, 30-34 parts of vermiculite powder, 26-30 parts of santal, 32-36 parts of lysimachia foenum-graecum powder, 26-30 parts of zinc sulfate, 30-34 parts of dipterex, 26-30 parts of chloral hydrate, 30-34 parts of sodium diacetate, 26-30 parts of butyl hydroxyanisole, 30-34 parts of sodium dehydroacetate, 26-30 parts of polyglycerol, 30-34 parts of olive oil macrogol ester, 26-30 parts of nimbin, 30-34 parts of buprofezin, 26-30 parts of rare earth cerium oxide powder, 30-34 parts of butene-fipronil, 26-30 parts of agritol, 30-34 parts of Dupont curzate, 26-30 parts of thiacloprid and 1000-2000 parts of water. The treating agent has excellent sterilization and mildew-proof performances, and raises the garden seed survival rate.
Owner:王璐
Method for determining the content of halogenated acids in chloral hydrate or its preparations
ActiveCN112345651BAvoid factors that interfere with test resultsEasy to operateComponent separationPhysical chemistryVapor phase chromatography
The application relates to a detection method for impurity content in medicines, in particular to a method for determining the content of halogenated acids in chloral hydrate or its preparations by gas chromatography.
Owner:TEFENG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com